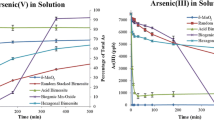
Abstract
Mining activities release arsenopyrite into calcareous soils where it undergoes weathering generating toxic compounds. The research evaluates the environmental impacts of these processes under semi-alkaline carbonated conditions. Electrochemical (cyclic voltammetry, chronoamperometry, EIS), spectroscopic (Raman, XPS), and microscopic (SEM, AFM, TEM) techniques are combined along with chemical analyses of leachates collected from simulated arsenopyrite weathering to comprehensively examine the interfacial mechanisms. Early oxidation stages enhance mineral reactivity through the formation of surface sulfur phases (e.g., S n 2−/S0) with semiconductor properties, leading to oscillatory mineral reactivity. Subsequent steps entail the generation of intermediate siderite (FeCO3)-like, followed by the formation of low-compact mass sub-micro ferric oxyhydroxides (α, γ-FeOOH) with adsorbed arsenic (mainly As(III), and lower amounts of As(V)). In addition, weathering reactions can be influenced by accessible arsenic resulting in the formation of a symplesite (Fe3(AsO4)3)-like compound which is dependent on the amount of accessible arsenic in the system. It is proposed that arsenic release occurs via diffusion across secondary α, γ-FeOOH structures during arsenopyrite weathering. We suggest weathering mechanisms of arsenopyrite in calcareous soil and environmental implications based on experimental data.










Similar content being viewed by others
References
Achiba WB, Gabteni N, Lakhdar A, Du Laing G, Verloo M, Jedidi N, Gallali T (2009) Effects of 5-year application of municipal solid waste compost on the distribution and mobility of heavy metals in a Tunisian calcareous soil. Agric Ecosyst Environ 130:156–163. doi:10.1016/j.agee.2009.01.001
Aguilar J, Dorronsoro C, Fernández E, Fernández J, García I, Martín F, Simón M (2004) Soil pollution by a pyrite mine spill in Spain: evolution in time. Environ Pollut 132:395–401. doi:10.1016/j.envpol.2004.05.028
Al TM, Martin CJ, Blowes DW (2000) Carbonate-mineral/water interactions in sulfide-rich mine tailings. Geochim Cosmochim Acta 64:3933–3948. doi:10.1016/S0016-7037(00)00483-X
Allison JD, Brown DS, Novogradac KJ (1991) MINTEQA2/PRODEFA2, a geochemical assessment model for environmental systems; version 3.11. EPA/600/3-91/021, Washington, DC, USA
Almeida CM, Giannetti BF (2003) The electrochemical behavior of pyrite–pyrrhotite mixtures. J Electroanal Chem 553:27–34. doi:10.1016/S0022-0728(03)00254-7
Armienta MA, Villasenor G, Rodriguez R, Ongley LK, Mango H (2001) The role of arsenic-bearing rocks in groundwater pollution at Zimapan Valley, Mexico. Environ Geol 40:571–581. doi:10.1007/s002540000220
ASTM (1999) Standard test method for shake extraction of solid waste with water D3987-85; West Conshocken
ASTM (2001) Standard test method for accelerated weathering of solid materials using a modified humidity cell D5744-96; West Conshocken
Ayame A, Uchida K, Iwataya M, Miyamoto M (2002) X-ray photoelectron spectroscopic study on α- and γ-bismuth molybdate surfaces exposed to hydrogen, propene and oxygen. Appl Catal A 227:7–17. doi:10.1016/S0926-860X(01)00918-8
Bard AJ, Parson R, Jordan J (1985) Standard potentials in aqueous solution. Marcel Decker, New York
Basu A, Schreiber ME (2013) Arsenic release from arsenopyrite weathering: insights from sequential extraction and microscopic studies. J Hazard Mater 262:896–904. doi:10.1016/j.jhazmat.2012.12.027
Bersani D, Lottici PP, Montenero A (1999) Micro‐Raman investigation of iron oxide films and powders produced by sol–gel syntheses. J Raman Spectrosc 30:355–360. doi:10.1002/(SICI)1097-4555(199905)30:5<355::AID-JRS398>3.0.CO;2-C
Bluteau MC, Becze L, Demopoulos GP (2009) The dissolution of scorodite in gypsum-saturated waters: evidence of Ca–Fe–AsO4 mineral formation and its impact on arsenic retention. Hydrometallurgy 97:221–227. doi:10.1016/j.hydromet.2009.03.009
Brock KJ (1972) Genesis of Garnet Hill Skarn, Calaveras County, California. Geol Soc Am Bull 83:3391–3404. doi:10.1130/0016-7606(1972)83[3391:GOGHSC]2.0.CO;2
Buckley AN, Walker GW (1998) The surface composition of arsenopyrite exposed to oxidizing environments. Appl Surf Sci 35:227–240
Buckley AN, Woods R (1987) The surface oxidation of pyrite. Appl Surf Sci 27:437–452
Burgot JL (2012) Ionic equilibria in analytical chemistry. New York, Springer
Cabrera-Sierra R, Hallen JM, Vazquez-Arenas J, Vazquez G, Gonzalez I (2010) EIS characterization of tantalum and niobium oxide films. J Electroanal Chem 638:51–58. doi:10.1016/j.jelechem.2009.10.021
Cabrera-Sierra R, Vazquez-Arenas J, Cardoso S, Luna-Sánchez RM, Trejo MA, Marín-Cruz J, Hallen JM (2011) Analysis of the formation of Ta2O5 passive films in acid media through mechanistic modeling. Electrochim Acta 56:8040–8047. doi:10.1016/j.electacta.2011.05.078
Caldeira CL, Ciminelli VST, Dias A, Osseo-Asare K (2003) Pyrite oxidation in alkaline solutions: nature of the product layer. Int J Miner Process 72:373–386. doi:10.1016/S0301-7516(03)00112-1
Caldeira CL, Ciminelli VS, Osseo-Asare K (2010) The role of carbonate ions in pyrite oxidation in aqueous systems. Geochim Cosmochim Acta 74:1777–1789. doi:10.1016/j.ca.2009.12.014
Castro-Larragoitia J, Kramar U, Puchelt H (1997) 200 years of mining activities at La Paz/San Luis Potosí/Mexico-consequences for environment and geochemical exploration. J Geochem Explor 58:81–91. doi:10.1016/S0375-6742(96)00054-4
Chappell DA, Craw D (2002) Geological analogue for circumneutral pH mine tailings: implications for long-term storage, Macraes Mine, Otago, New Zealand. Appl Geochem 17:1105–1114. doi:10.1016/S0883-2927(02)00002-1
Chen Y, Ahsan H (2004) Cancer burden from arsenic in drinking water in Bangladesh. Am J Public Health 94:741–744. doi:10.2105/AJPH.94.5.741
Cheng H, Hu Y, Luo J, Xu B, Zhao J (2008) Geochemical processes controlling fate and transport of arsenic in acid mine drainage (AMD) and natural systems. J Hazard Mater 165:13–26. doi:10.1016/j.hazmat.2008.10.070
Chiprés JA, Salinas JC, Castro-Larragoitia J, Monroy M (2008) Geochemical mapping of major and trace elements in soils from the Altiplano Potosino, Mexico: a multi-scale comparison. Geochem Explor Environ Anal 8:279–290. doi:10.1144/1467-7873/08-181
Choo CO, Lee JK (2002) Mineralogical and geochemical controls on the formation of schwertmannite and goethite in the wetland at Dalseong tungsten mine, Korea. Geosci J 6:281–287. doi:10.1007/BF03020613
Ciminelli VST, Osseo-Asare K (1995) Kinetics of pyrite oxidation in sodium carbonate solutions. Metall Mater Trans B 26:209–218. doi:10.1007/BF02660961
Cisneros-González I, Oropeza-Guzmán MT, González I (2000) An electrochemical study of galena concentrate in perchlorate medium at pH 2.0: the influence of chloride ions. Electrochim Acta 45:2729–2741. doi:10.1016/S0013-4686(00)00392-3
Clesceri LS, Greenberg AE, Eaton AD (1998) Standard methods for the examination of water and wastewater, method no: 2320B-acidity or total alkalinity in water, 20th edn. Port City Press, Baltimore
Constantin CA, Chiriţă P (2013) Oxidative dissolution of pyrite in acidic media. J Appl Electrochem 43:659–666. doi:10.1007/s10800-013-0557-y
Costa MC, Do Rego AB, Abrantes LM (2002) Characterization of a natural and an electro-oxidized arsenopyrite: a study on electrochemical and X-ray photoelectron spectroscopy. Int J Miner Process 65:83–108. doi:10.1016/S0301-7516(01)00059-X
Courtin-Nomade A, Bril H, Neel C, Lenain JF (2003) Arsenic in iron cements developed within tailings of a former metalliferous mine-Enguialès, Aveyron, France. Appl Geochem 18:395–408. doi:10.1016/S0883-2927(02)00098-7
Craw D, Chappell D, Nelson M, Walrond M (1999) Consolidation and incipient oxidation of alkaline arsenopyrite-bearing mine tailings, Macraes Mine, New Zealand. Appl Geochem 14:485–498. doi:10.1016/S0883-2927(98)00063-8
Craw D, Koons PO, Chappell DA (2002) Arsenic distribution during formation and capping of an oxidised sulphidic minesoil, Macraes mine, New Zealand. J Geochem Explor 76:13–29. doi:10.1016/S0375-6742(02)00202-9
Craw D, Falconer D, Youngson JH (2003) Environmental arsenopyrite stability and dissolution: theory, experiment, and field observations. Chem Geol 199:71–82. doi:10.1016/S0009-2541(03)00117-7
Cruz R, Bertrand V, Monroy M, González I (2001a) Effect of sulfide impurities on the reactivity of pyrite and pyritic concentrates: a multi-tool approach. Appl Geochem 16:803–819. doi:10.1016/S0883-2927(00)00054-8
Cruz R, Méndez BA, Monroy M, González I (2001b) Cyclic voltammetry applied to evaluate reactivity in sulfide mining residues. Appl Geochem 16:1631–1640. doi:10.1016/S0883-2927(01)00035-X
Cruz R, González I, Monroy M (2005) Electrochemical characterization of pyrrhotite reactivity under simulated weathering conditions. Appl Geochem 20:109–121. doi:10.1016/j.apgeochem.2004.07.007
Das S, Hendry MJ (2011) Application of Raman spectroscopy to identify iron minerals commonly found in mine wastes. Chem Geol 290:101–108. doi:10.1016/j.chemgeo.2011.09.001
De Faria DLA, Silva SV, De Oliveira MT (1997) Raman microspectroscopy of some iron oxides and oxyhydroxides. J Raman Spectrosc 28:873–878. doi:10.1002/(SICI)1097-4555(199711)28:11<873::AID-JRS177>3.0.CO;2-B
De Levie R (2003) The Henderson-Hasselbalch equation: its history and limitations. J Chem Educ 80:146. doi:10.1021/ed080p146
Déscostes M, Beaucaire C, Mercier F, Savoye F, Sow J, Zuddas P (2002) Effect of carbonate ions on pyrite (FeS2) dissolution. Bull Soc Geol France 173:265–270. doi:10.2113/173.3.265
Dixit S, Hering JG (2003) Comparison of arsenic (V) and arsenic (III) sorption onto iron oxide minerals: implications for arsenic mobility. Environ Sci Technol 37:4182–4189. doi:10.1021/es030309t
Dorronsoro C, Martin F, Ortiz I, García M, Simón E, Fernández E, Aguilar J, Fernández J (2002) Migration of trace elements from pyrite tailings in carbonate soils. J Environ Qual 31:829–835. doi:10.2134/jeq2002.8290
Drzyzga M, Szade J, Deniszczyk J, Michalecki T (2003) Electronic structure of rare earth bismuthides. J Phys Condens Matter 15:3701. doi:10.1088/0953-8984/15/22/305
Eggleston CM, Ehrhardt JJ, Stumm W (1996) Surface structural controls on pyrite oxidation kinetics: an XPS-UPS, STM, and modeling study. Am Mineral 81:1036–1056
El Jaroudi O, Picquenard E, Demortier A, Leliuer JP, Corset J (1999) Polysulfide anions. 1. Structure and vibrational spectra of the S2 2− and S3 2− anions. Influence of the cations on bond length and angle. Inorg Chem 38:2394–2401. doi:10.1021/ic9811143
El Jaroudi O, Picquenard E, Demotier A, Leliuer JP, Corset J (2000) Polysulfide anions II: structure and vibrational spectra of the S4 2− and S5 2− anions. Influence of the cations on bond length, valence and torsion angle. Inorg Chem 39:2593–2603. doi:10.1021/ic991419x
Evangelou VP, Zhang YL (1995) A review: pyrite oxidation mechanisms and acid mine drainage prevention. Crit Rev Environ Sci Technol 25:141–199. doi:10.1080/10643389509388477
Evangelou VP, Seta AK, Holt A (1998) Potential role of bicarbonate during pyrite oxidation. Environ Sci Technol 32:2084–2091. doi:10.1021/es970829m
Fujita T, Taguchi R, Abumiya M, Matsumoto M, Shibata E, Nakamura T (2008) Novel atmospheric scorodite synthesis by oxidation of ferrous sulfate solution. Part I. Hydrometallurgy 90:92–102. doi:10.1016/j.hydromet.2007.09.012
García-Meza JV, Contreras-Aganza MI, Castro-Larragoitia J, Lara RH (2011) Growth of photosynthetic biofilms and Fe, Pb, Cu, and Zn speciation in unsaturated columns with calcareous mine tailings from arid zones. Appl Environ Soil Sci. doi:10.1155/2011/732984
García-Meza JV, Lara RH, Navarro-Contreras HR (2012) Application of Raman spectroscopy to the biooxidation analysis of sulfide minerals. Int J Spectrosc. doi:10.1155/2012/501706
Gas’kova OL, Shironosova GP, Bortnikova SB (2008) Thermodynamic estimation of the stability field of bukovskyite, an iron sulfoarsenate. Geochem Int 46:85–89. doi:10.1134/S0016702908010072
Gehring AU, Hofmeister AM (1994) The transformation of lepidocrocite during heating: a magnetic and spectroscopic study. Clay Clay Miner 42:409–415
Giannetti BF, Almeida CMVB, Bonilla SH (2006) Electrochemical kinetic study of surface layer growth on natural pyrite in acid medium. Colloids Surf A 272:130–138. doi:10.1016/j.colsurfa.2005.07.018
Goldberg S, Johnston CT (2001) Mechanisms of arsenic adsorption on amorphous oxides evaluated using macroscopic measurements, vibrational spectroscopy, and surface complexation modeling. J Colloid Interface Sci 234:204–216. doi:10.1006/jcis.2000.7295
Grift B, Griffioen J (2008) Modelling assessment of regional groundwater contamination due to historic smelter emissions of heavy metals. J Contam Hydrol 96:48–68
Gu GH, Sun XJ, Hu KT, Li JH, Qiu GZ (2012) Electrochemical oxidation behavior of pyrite bioleaching by Acidthiobacillus ferrooxidans. Trans Nonferrous Metals Soc China 22:1250–1254. doi:10.1016/S1003-6326(11)61312-5
Hamilton IC, Woods R (1981) An investigation of surface oxidation of pyrite and pyrrhotite by linear potential sweep voltammetry. J Electroanal Chem 118:327–343. doi:10.1016/S0022-0728(81)80551-7
Hiller E, Petrák M, Tóth R, Lalinská-Voleková B, Jurkovič L, Kučerová G, Radková A, Šottník P, Vozár J (2013) Geochemical and mineralogical characterization of a neutral, low-sulfide/high-carbonate tailings impoundment, Markušovce, eastern Slovakia. Environ Sci Pollut Res 11:7627–7642. doi:10.1007/s11356-013-1581-5
Hossner LR, Doolittle JJ (2003) Iron sulfide oxidation as influenced by calcium carbonate application. J Environ Qual 32:773–780. doi:10.2134/jeq2003.7730
Jiménez-Cárceles FJ, Álvarez-Rogel J, Conesa HR (2008) Trace element concentrations in saltmarsh soils strongly affected by wastes from metal sulphide mining areas. Water Air Soil Pollut 188:283–295. doi:10.1007/s11270-007-9544-4
Jönsson J, Persson P, Sjöberg S, Lövgren L (2005) Schwertmannite precipitated from acid mine drainage: phase transformation, sulphate release and surface properties. Appl Geochem 20:179–191. doi:10.1016/j.apgeochem.2004.04.008
Jönsson J, Jönsson J, Lövgren L (2006) Precipitation of secondary Fe(III) minerals from acid mine drainage. Appl Geochem 21:437–445. doi:10.1016/j.apgeochem.2005.12.008
Jurjovec J, Ptacek CJ, Blowes DW (2002) Acid neutralization mechanisms and metal release in mine tailings: a laboratory column experiment. Geochim Cosmochim Acta 66:1511–1523. doi:10.1016/S0016-7037(01)00874-2
Karthe S, Szargan R, Suoninen E (1993) Oxidation of pyrite surfaces: a photoelectron spectroscopic study. Appl Surf Sci 72:157–170. doi:10.1016/0169-4332(93)90007-X
Kelsall GH, Yin Q, Vaughan DJ, England KER, Brandon NP (1999) Electrochemical oxidation of pyrite (FeS2) in aqueous electrolytes. J Electroanal Chem 471:116–125. doi:10.1016/S0022-0728(99)00261-2
Kim MJ, Nriagu J, Haack S (2000) Carbonate ions and arsenic dissolution by groundwater. Environ Sci Technol 34:3094–3100. doi:10.1021/es990949p
Klein CB, Leszczynska J, Hickey C, Rossman TG (2007) Further evidence against a direct genotoxic mode of action for arsenic-induced cancer. Toxicol Appl Pharmacol 222:289–297. doi:10.1016/j.taap.2006.12.033
Knipe SW, Mycroft JR, Pratt AR, Nesbitt HW, Bancroft GM (1995) X-ray photoelectron spectroscopic study of water adsorption on iron sulphide minerals. Geochim Cosmochim Acta 59:1079–1090. doi:10.1016/0016-7037(95)00025-U
Kossoff D, Hudson-Edwards KA, Dubbin WE, Alfredsson M, Geraki T (2012) Cycling of As, P, Pb and Sb during weathering of mine tailings: implications for fluvial environments. Mineral Mag 76:1209–1228. doi:10.1180/minmag.2012.076.5.14
Koura N, Kohara S, Takeuchi K, Takahashi S, Curtiss LA, Grimsditch M, Saboungi ML (1996) Alkali carbonates: Raman spectroscopy, ab initio calculations, and structure. J Mol Struct 382:163–169. doi:10.1016/0022-2860(96)09314-3
Krause E, Ettel VA (1988) Solubility and stability of scorodite, FeAsO4 · 2H2O: new data and further discussion. Am Mineral 73:850–854
Labastida I, Armienta MA, Lara-Castro RH, Aguayo A, Cruz O, Ceniceros N (2013) Treatment of mining acidic leachates with indigenous limestone, Zimapan Mexico. J Hazard Mater 262:1187–1195. doi:10.1016/j.jhazmat.2012.07.006
Langmuir D (1997) Environmental geochemistry. Ed. Prentice Hall, USA, p 73
Langmuir D, Mahoney J, Rowson J (2006) Solubility products of amorphous ferric arsenate and crystalline scorodite (FeAsO4·2H2O) and their application to arsenic behavior in buried mine tailings. Geochim Cosmochim Acta 70:2942–2956. doi:10.1016/j.gca.2006.03.006
Lara RH, Monroy MG, Mallet M, Dossot M, González MA, Cruz R (2015a) An experimental study of iron sulfides weathering under simulated calcareous soil conditions. Environ Earth Sci 73:1849–1869. doi:10.1007/s12665-014-3540-y
Lara RH, Vazquez-Arenas JG, Ramos-Sanchez G, Galvan M, Lartundo-Rojas L (2015b) Experimental and theoretical analysis accounting for differences of pyrite and chalcopyrite oxidative behaviors for prospective environmental and (Bio)leaching applications. J Phys Chem C 19:18364–18379. doi:10.1021/acs.jpcc.5b05149
Lázaro I, Martínez-Medina N, Rodríguez I, Arce E, González I (1995) The use of carbon pate electrodes with non-conducting binder for the study of minerals: chalcopyrite. Hydrometallurgy 38:275–285. doi:10.1016/0304-386X(94)00070-J
Lehner S, Ciobanu M, Savage K, Cliffel DE (2008) Electrochemical impedance spectroscopy of synthetic pyrite doped with As, Co, and Ni. J Electrochem Soc 155:61–70. doi:10.1149/1.2885103
Lin CC, Liu LG (1997) Post-aragonite phase transitions in strontianite and cerussite-a high-pressure Raman spectroscopic study. J Phys Chem Solids 58:977–987. doi:10.1016/S0022-3697(96)00201-6
Lin HK, Say WC (1999) Study of pyrite oxidation by cyclic voltammetric, impedance spectroscopic and potential step techniques. J Appl Electrochem 29:987–994. doi:10.1023/A:1003578728263
Lin HT, Wang MC, Seshaiah K (2008) Mobility of adsorbed arsenic in two calcareous soils as influenced by water extract of compost. Chemosphere 71:742–749. doi:10.1016/j.chemosphere.2007.10.022
Liu Y, Dang Z, Wu P, Lu J, Shu X, Zheng L (2011a) Influence of ferric iron on the electrochemical behavior of pyrite. Ionics 17:169–176. doi:10.1007/s11581-010-0492-4
Liu Y, Dang Z, Lu G, Wu P, Feng C, Yi X (2011b) Utilization of electrochemical impedance spectroscopy for monitoring pyrite oxidation in the presence and absence of Acidithiobacillus ferrooxidans. Miner Eng 24:833–838. doi:10.1016/j.mineng.2011.03.002
Lu P, Zhu C (2011) Arsenic Eh–pH diagrams at 25 C and 1 bar. Environ Earth Sci 62:1673–1683. doi:10.1007/s12665-010-0652-x
Majzlan J, Grevel KD, Navrotsky A (2003) Thermodynamics of Fe oxides: part II. Enthalpies of formation and relative stability of goethite (α-FeOOH), lepidocrocite (γ-FeOOH), and maghemite (γ-Fe2O3). Am Mineral 88:855–859
Martín F, Diez M, García I, Simón M, Dorronsoro C, Iriarte A, Aguilar J (2007) Weathering of primary minerals and mobility of major elements in soils affected by an accidental spill of pyrite tailing. Sci Total Environ 378:49–52. doi:10.1016/j.scitotenv.2007.01.031
Martín F, García I, Díez M, Sierra M, Simon M, Dorronsoro C (2008) Soil alteration by continued oxidation of pyrite tailings. Appl Geochem 23:1152–1165. doi:10.1016/j.apgeochem.2007.11.012
Martínez-Sánchez MJ, Navarro MC, Pérez-Sirvent C, Marimón J, Vidal J, García-Lorenzo ML, Bech J (2008) Assessment of the mobility of metals in a mining-impacted coastal area (Spain, Western Mediterranean). J Geochem Explor 96:171–182. doi:10.1016/j.gexplo.2007.04.006
Martínez-Villegas N, Briones-Gallardo R, Ramos-Leal JA, Avalos-Borja M, Castañón-Sandoval AD, Razo-Flores E, Villalobos M (2013) Arsenic mobility controlled by solid calcium arsenates: a case study in Mexico showcasing a potentially widespread environmental problem. Environ Pollut 176:114–122. doi:10.1016/j.envpol.2012.12.025
McGuire MM, Jallad KN, Ben-Amotz D, Hamers RJ (2001) Chemical mapping of elemental sulfur on pyrite and arsenopyrite surfaces using near-infrared Raman imaging microscopy. Appl Surf Sci 178:105–115. doi:10.1016/S0169-4332(01)00303-8
Mehmood A, Hayat R, Wasim M, Akhtar MS (2009) Mechanisms of arsenic adsorption in calcareous soils. J Agric Biol Sci 1:59–65
Meléndez AM, Arroyo R, González I (2010) On the reactivity of sulfosalts in cyanide aqueous media: structural, bonding and electronic aspects. Chem Phys Chem 11:2879–2886. doi:10.1002/cphc.201000187
Mikhlin YL (2000) Reactivity of pyrrhotite surfaces: an electrochemical study. Phys Chem Chem Phys 2:5672–5677. doi:10.1039/B005373M
Mikhlin YL, Romanchenko AS (2007) Gold deposition on pyrite and the common sulfide minerals: an STM/STS and SR-XPS study of surface reactions and Au nanoparticles. Geochim Cosmochim Acta 71:5985–6001. doi:10.1016/j.gca.2007.10.001
Mikhlin YL, Tomashevich YV, Pashkov GL, Okotrub AV, Asanov IP, Mazalov LN (1998) Electronic structure of the non-equilibrium iron-deficient layer of hexagonal pyrrhotite. Appl Surf Sci 125:73–84. doi:10.1016/S0169-4332(97)00386-3
Mikhlin YL, Tomashevich YV, Asanov IP, Okotrub AV, Varnek VA, Vyalikh DV (2004) Spectroscopic and electrochemical characterization of the surface layers of chalcopyrite (CuFeS2) reacted in acidic solutions. Appl Surf Sci 225:395–409. doi:10.1016/j.apsusc.2003.10.030
Molinari A, Ayora C, Marcaccio M, Guadagnini L, Sanchez-Vila X, Guadagnini A (2014) Geochemical modeling of arsenic release from a deep natural solid matrix under alternated redox conditions. Environ Sci Pollut Res 21:1628–1637. doi:10.1007/s11356-013-2054-6
Moreno-Medrano ED, Casillas N, Cruz R, Lara-Castro RH, Bárcena-Soto M, Larios-Durán ER (2011) Impedance study during anodic oxidation of native galena in a highly concentrated xanthate solution. Int J Electrochem Sci 6:6319–6331
Morgan B, Lahav O (2007) The effect of pH on the kinetics of spontaneous Fe(II) oxidation by O2 in aqueous solution–basic principles and a simple heuristic description. Chemosphere 68:2080–2084. doi:10.1016/j.chemosphere.2007.02.015
Mullet M, Demoisson F, Bernard H, Michot L, Vantelon D (2006) Aqueous Cr(VI) reduction by pyrite: speciation and characterization of the solid phases by X-ray photoelectron, Raman and X-ray absorption spectroscopies. Geochim Cosmochim Acta 71:3257–3271. doi:10.1016/j.gca.2006.09.008
Murciego A, Alvarez-Ayuso E, Pellitero E, Rodríguez M, García-Sánchez A, Tamayo A, Rubin J (2011) Study of arsenopyrite weathering products in mine wastes from abandoned tungsten and tin exploitations. J Hazard Mater 186:590–601. doi:10.1016/j.jhazmat.2010.11.033
Murphy R, Strongin DR (2009) Surface reactivity of pyrite and related sulfides. Surf Sci Rep 64:1–45. doi:10.1016/j.surfrep.2008.09.002
Mycroft JR, Bancroft GM, McIntyre NS, Lorimer JW, Hill IR (1990) Detection of sulphur and polysulphides on electrochemically oxidized pyrite surfaces by X-ray photoelectron spectroscopy and Raman spectroscopy. J Electroanal Chem 292:139–152
Myneni SC, Traina SJ, Waychunas GA, Logan TJ (1998) Experimental and theoretical vibrational spectroscopic evaluation of arsenate coordination in aqueous solutions, solids, and at mineral-water interfaces. Geochim Cosmochim Acta 62:3285–3300. doi:10.1016/S0016-7037(98)00222-1
Nan Z, Zhao C (2000) Heavy metal concentrations in gray calcareous soils of Baiyin region, Gansu province, PR China. Water Air Soil Pollut 118:131–142. doi:10.1023/A:1005135618750
Nava D, González I, Leinen D, Ramos-Barrado JR (2008) Surface characterization by X-ray photoelectron spectroscopy and cyclic voltammetry of products formed during the potentiostatic reduction of chalcopyrite. Electrochim Acta 53:4889–4899. doi:10.1016/j.electacta.2008.01.088
Navarro MC, Pérez-Sirvent C, Martínez-Sánchez MJ, Vidal J, Tovar PJ, Bech J (2008) Abandoned mine sites as a source of contamination by heavy metals: a case study in a semi-arid zone. J Geochem Explor 96:183–193. doi:10.1016/j.gexplo.2007.04.011
Nesbitt HW, Muir IJ (1994) X-ray photoelectron spectroscopic study of a pristine pyrite surface reacted with water vapour and air. Geochim Cosmochim Acta 54:4667–4679
Nesbitt HW, Muir IJ (1998) Oxidation states and speciation of secondary products on pyrite and arsenopyrite reacted with mine waste waters and air. Miner Petrol 62:123–144. doi:10.1007/BF01173766
Nesbitt HW, Muir IJ, Pratt AR (1995) Oxidation of arsenopyrite by air and air-saturated, distilled water, and implications for mechanism of oxidation. Geochim Cosmochim Acta 59:1773–1786. doi:10.1016/0016-7037(95)00081-A
Nesbitt HW, Bancroft GM, Pratt AR, Scaini MJ (1998) Sulfur and iron surface states on fractured pyrite surfaces. Am Miner 83:1067–1076
Nesbitt H, Scaini M, Hoechst H, Brancroft GM, Schaufuss AG, Szargan R (2000) Synchrotron XPS evidence for Fe2+–S and Fe3+–S surface species on pyrite fracture surfaces and their 3d electronic states. Am Mineral 85:850–857
Nicholson RV, Gillham RW, Reardon EJ (1988) Pyrite oxidation in carbonate-buffered solution: 1. Experimental kinetics. Geochim Cosmochim Acta 52:1077–1085
Nicholson RV, Gillham RW, Reardon EJ (1990) Pyrite oxidation in carbonate-buffered solution: 2. Rate control by oxide coatings. Geochim Cosmochim Acta 54:395–402
Nowak P, Laajalehto K (2000) Oxidation of galena surface-an XPS study of the formation of sulfoxy species. Appl Surf Sci 157:101–111. doi:10.1016/S0169-4332(99)00575-9
Ona-Nguema G, Morin G, Juillot F, Calas G, Brown GE (2005) EXAFS analysis of arsenite adsorption onto two-line ferrihydrite, hematite, goethite, and lepidocrocite. Environ Sci Technol 39:9147–9155. doi:10.1021/es050889p
Paktunc D, Dutrizac J, Gertsman V (2008) Synthesis and phase transformations involving scorodite, ferric arsenate and arsenical ferrihydrite: implications for arsenic mobility. Geochim Cosmochim Acta 72:2649–2672. doi:10.1016/j.gca.2008.03.012
Pappu A, Saxena M, Asolekar SR (2006) Jarosite characteristics and its utilisation potentials. Sci Total Environ 359:232–243. doi:10.1016/j.scitotenv.2005.04.024
Parker GK, Woods R, Hope GA (2008) Raman investigation of chalcopyrite oxidation. Colloids Surf A 318:160–168. doi:10.1016/j.colsurfa.2007.12.030
Perfetti E, Pokrovski GS, Ballerat-Busserolles K, Majer V, Gibert F (2008) Densities and heat capacities of aqueous arsenious and arsenic acid solutions to 350°C and 300 bar, and revised thermodynamic properties of, and iron sulfarsenide minerals. Geochim Cosmochim Acta 72:713–731. doi:10.1016/j.gca.2007.11.017
Pratt AR, Muir IJ, Nesbitt HW (1994) X-ray photoelectron and Auger electron spectroscopic studies of pyrrhotite and mechanism of air oxidation. Geochim Cosmochim Acta 58:827–841
Pratt AR, McIntyre NS, Splinter SJ (1998) Deconvolution of pyrite, marcasite and arsenopyrite XPS spectra using the maximum entropy method. Surf Sci 396:266–272. doi:10.1016/S0039-6028(97)00675-4
Raposo JC, Zuloaga O, Sanz J, Villanueva U, Crea P, Etxebarria N, Madariaga JM (2006) Analytical and thermodynamical approach to understand the mobility/retention of arsenic species from the river to the estuary. The Bilbao case study. Mar Chem 99:42–51. doi:10.1016/j.marchem.2005.02.004
Razo I, Carrizales L, Castro J, Díaz-Barriga F, Monroy M (2004) Arsenic and heavy metal pollution of soil, water and sediments in a semi-arid climate mining area in Mexico. Water Air Soil Pollut 152:129–152. doi:10.1023/B:WATE.0000015350.14520.c1
Richardson S, Vaughan DJ (1989) Arsenopyrite: a spectroscopic investigation of altered surfaces. Mineral Mag 53:223–229
Robins RG (1981) The solubility of metal arsenates. Metall Trans B 12:103–109. doi:10.1007/BF02674763
Romero FM, Armienta MA, Villaseñor G, González JL (2006) Mineralogical constraints on the mobility of arsenic in tailings from Zimapán, Hidalgo, Mexico. Int J Environ Pollut 26:23–40. doi:10.1504/IJEP.2006.009097
Romero FM, Nunez L, Gutiérrez ME, Armienta MA, Ceniceros-Gómez AE (2011) Evaluation of the potential of indigenous calcareous shale for neutralization and removal of arsenic and heavy metals from acid mine drainage in the Taxco Mining Area, Mexico. Arch Environ Contam Toxicol 60:191–203. doi:10.1007/s00244-010-9544-z
Sahoo PK, Tripathy S, Panigrahi MK, Equeenuddin SMD (2012) Mineralogy of Fe-precipitates and their role in metal retention from an acid mine drainage site in India. Mine Water Environ 31:344–352. doi:10.1007/s10230-012-0203-7
Samadi A, Gilkes RJ (1999) Phosphorus transformations and their relationships with calcareous soil properties of Southern Western Australia. Soil Sci Soc Am J 63:809–815. doi:10.2136/sssaj1999.634809x
Sandström Å, Shchukarev A, Paul J (2005) XPS characterisation of chalcopyrite chemically and bio-leached at high and low redox potential. Miner Eng 18:505–515. doi:10.1016/j.mineng.2004.08.004
Sasaki K, Tsunekawa M, Ohtsuka T, Konno H (1998) The role of sulfur-oxidizing bacteria Thiobacillus thiooxidans in pyrite weathering. Colloids Surf A 133:269–278. doi:10.1016/S0927-7757(97)00200-8
Savage KS, Bird DK, O'Day PA (2005) Arsenic speciation in synthetic jarosite. Chem Geol 215:473–498. doi:10.1016/j.chemgeo.2004.06.046
Schaufuß AG, Nesbitt HW, Kartio I, Laajalehto K, Bancroft GM, Szargan R (1998a) Incipient oxidation of fractured pyrite surfaces in air. J Electron Spectrosc Relat Phenom 96:69–82. doi:10.1016/S0368-2048(98)00237-0
Schaufuss AG, Nesbitt HW, Kartio I, Laajalehto K, Bancroft GM, Szargan R (1998b) Reactivity of surface chemical states on fractured pyrite. Surf Sci 411:321–325. doi:10.1016/S0039-6028(98)00355-0
Schaufuss AG, Nesbitt HW, Scaini MJ, Hoechst H, Bancroft MG, Szargan R (2000) Reactivity of surface sites on fractured arsenopyrite (FeAsS) toward oxygen. Am Miner 85:1754–1766
Sengupta AK (2001) Environmental separation of heavy metals: engineering processes. CRC Press
Shi J, Li T, Feng M, Mao Z, Wang C (2006) Study of the corrosion from the printing plates of ‘Guan Zi’ by Raman spectroscopy. J Raman Spectrosc 37:836–840. doi:10.1002/jrs.1511
Shim SH, Duffy TS (2001) Raman spectroscopy of Fe2O3 to 62 GPa. Am Miner 87:318–326
Singhania S, Wang Q, Filippou D, Demopoulos GP (2006) Acidity, valency and third-ion effects on the precipitation of scorodite from mixed sulfate solutions under atmospheric-pressure conditions. Metall Mater Trans B 37:189–197. doi:10.1007/BF02693148
Skousen J, Rose A, Geidel G, Foreman J, Evans R, Hellier and Members of the Avoidance and Remediation Working Group of the Acid Drainage Technology Initiative (1998) A handbook of technologies for avoidance and remediation of acid mine drainage, the national mine land reclamation center. National Mine Land Reclamation Center PBS, Virginia, pp 10–11
Sojdehee M, Rasa I, Nezafati N, Abedini MV, Madani N, Zeinedini E (2015) Probabilistic modeling of mineralized zones in Daralu copper deposit (SE Iran) using sequential indicator simulation. Arab J Geosci. doi:10.1007/s12517-015-1828-1
Stumm W, Morgan JJ (1996) Aquatic chemistry: chemical equilibria and rates in natural water, 3rd edn. Wiley, New York
Takahashi M, Noda M, Okuyama M (2003) Photoelectron spectroscopic studies of bismuth-excess strontium bismuth tantalate thin films and their high-pressure-O2-annealing effects. J Appl Phys 94:6729–6734. doi:10.1063/1.1621737
Thomas JE, Skinner WM, Smart RS (2003) A comparison of the dissolution behavior of troilite with other iron (II) sulfides; implications of structure. Geochim Cosmochim Acta 67:831–843. doi:10.1016/S0016-7037(02)01146-8
Toniazzo V, Mustin C, Portal JM, Humbert B, Benoit R, Erre R (1999) Elemental sulfur at the pyrite surfaces: speciation and quantification. Appl Surf Sci 143:229–237. doi:10.1016/S0169-4332(98)00918-0
Turcotte RE, Benner AM (1993) Surface analysis of electrochemically oxidized metal sulfides using Raman spectroscopy. J Electroanal Chem 347:195–919. doi:10.1016/0022-0728(93)80088-Y
Velasquez P, Leinen D, Pascual J, Ramos-Barrado JR, Grez P, Gomez H, Cordova R (2005) A chemical, morphological, and electrochemical (XPS, SEM/EDX, CV, and EIS) analysis of electrochemically modified electrode surfaces of natural chalcopyrite (CuFeS2) and pyrite (FeS2) in alkaline solutions. J Phys Chem B 109:4977–4988. doi:10.1021/jp048273u
Villenuéve M (2002) Évaluation du comportement géochimique á long terme de rejetsminiers à faible potentiel de génération d’acide á l’aide d’essais cinétiques. Msc Thesis, Université de Montreal
Walker DJ, Clemente R, Roig A, Bernal MP (2003) The effects of soil amendments on heavy metal bioavailability in two contaminated Mediterranean soils. Environ Pollut 122:303–312. doi:10.1016/S0269-7491(02)00287-7
Walker FP, Schreiber ME, Rimstidt JD (2006) Kinetics of arsenopyrite oxidative dissolution by oxygen. Geochim Cosmochim Acta 70:1668–1676. doi:10.1016/j.gca.2005.12.010
Walsh A, Watson GW, Payne DJ, Edgell RG, Guo J, Glans PA, Learmonth T, Smith KE (2006) Electronic structure of the α and δ phases of Bi2O3: a combined ab initio and x-ray spectroscopy study. Phys Rev B 73:235104. doi:10.1103/PhysRevB.73.235104
Wang Y, Morin G, Ona-Nguema G, Brown GE Jr (2014) Arsenic(III) and arsenic(V) speciation during transformation of lepidocrocite to magnetite. Environ Sci Technol 48:14282–14290. doi:10.1021/es5033629
Xia L, Yin C, Dai S, Qiu G, Chen X, Liu J (2010) Bioleaching of chalcopyrite concentrate using Leptospirillum ferriphilum, Acidithiobacillus ferrooxidans and Acidithiobacillus thiooxidans in a continuous bubble column reactor. J Ind Microbiol Biotechnol 37:289–295. doi:10.1007/s10295-009-0672-2
Acknowledgments
Financial support for this research work is greatly appreciated from CONACYT (Grants 2012-183230 and 2013-205416). The authors thank Jacques Lambert (Université de Lorraine), Erasmo Mata (IG-UASLP), and Francisco Galindo (IM-UASLP) for their XPS analyses and massive mineral electrodes preparation and construction, respectively. The authors also thank Pr. Marcos G. Monroy (IM-UASLP) for invaluable support during this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interest
The authors declare no competing financial interest.
Additional information
Responsible editor: Zhihong Xu
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 1150 kb)
Rights and permissions
About this article
Cite this article
Lara, R.H., Velázquez, L.J., Vazquez-Arenas, J. et al. Arsenopyrite weathering under conditions of simulated calcareous soil. Environ Sci Pollut Res 23, 3681–3706 (2016). https://doi.org/10.1007/s11356-015-5560-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-015-5560-x