Abstract
Introduction
The study of natural variation of metabolites brings valuable information on the physiological state of the organisms as well as their phenotypic traits. In marine organisms, metabolome variability has mostly been addressed through targeted studies on metabolites of ecological or pharmaceutical interest. However, comparative metabolomics has demonstrated its potential to address the overall and complex metabolic variability of organisms.
Objectives
In this study, the intraspecific (temporal and spatial) variability of two Mediterranean Haliclona sponges (H. fulva and H. mucosa) was investigated through an untargeted and then targeted metabolomics approach and further compared to their interspecific variability.
Methods
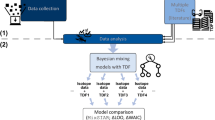
Samples of both species were collected monthly during 1 year in the coralligenous habitat of the Northwestern Mediterranean sae at Marseille and Nice. Their metabolomic profiles were obtained by UHPLC-QqToF analyses.
Results
Marked variations were noticed in April and May for both species including a decrease in Shannon’s diversity and concentration in specialized metabolites together with an increase in fatty acids and lyso-PAF like molecules. Spatial variations across different sampling sites could also be observed for both species, however in a lesser extent.
Conclusions
Synchronous metabolic changes possibly triggered by physiological factors like reproduction and/or environmental factors like an increase in the water temperature were highlighted for both Mediterranean Haliclona species inhabiting close habitats but displaying different biosynthetic pathways. Despite significative intraspecific variations, metabolomic variability remains minor when compared to interspecific variations for these congenerous species, therefore suggesting the predominance of genetic information of the holobiont in the observed metabolome.






Similar content being viewed by others
References
Abdo, D. A., Motti, C. A., Battershill, C. N., & Harvey, E. S. (2007). Temperature and spatiotemporal variability of salicylihalamide a in the sponge Haliclona sp. Journal of Chemical Ecology, 33, 1635–1645.
Agrawal, A. A., Hastings, A. P., Johnson, M. T. J., Maron, J. L., & Salminen, J.-P. (2012). Insect herbivores drive real-time ecological and evolutionary change in plant populations. Science, 338, 113–116.
Alam, N., Bae, B. H., Hong, J., Lee, C.-O., Shin, B. A., Im, K. S., & Jung, J. H. (2001). Additional bioactive lyso-PAF congeners from the sponge Spirastrella abata. Journal of Natural Products, 64, 533–535.
Alarif Walied, M., Abdel-Lateff, A., Al-Lihaibi Sultan, S., Seif-Eldin, A., N. & Badria Farid, A. (2013). A new cytotoxic brominated acetylenic hydrocarbon from the marine sponge Haliclona sp. with a selective effect against human breast cancer. Zeitschrift für Naturforschung C, 68, 70–75.
Aoki, N., Yamamoto, K., Ogawa, T., Ohta, E., Ikeuchi, T., Kamemura, K., Ikegami, S., & Ohta, S. (2013). Bromotheoynic acid, a brominated acetylenic acid from the marine sponge Theonella swinhoei. Natural Product Research, 27, 117–122.
Aratake, S., Trianto, A., Hanif, N., De Voogd, N. J., & Tanaka, J. (2009). A new polyunsaturated brominated fatty acid from a Haliclona sponge. Marine Drugs, 7, 523–527.
Bergquist, P. R., & Hartman, W. D. (1969). Free amino acid patterns and the classification of the demospongiae. Marine Biology, 3, 247–268.
Blunt, J. W., Copp, B. R., Keyzers, R. A., Munro, M. H. G., & Prinsep, M. R. (2017). Marine natural products. Natural Product Reports, 34, 235–294.
Borchert, E., Jackson, S. A., O’gara, F., & Dobson, A. D. W. (2016). Diversity of natural product biosynthetic genes in the microbiome of the deep sea sponges Inflatella pellicula, Poecillastra compressa, and Stelletta normani. Frontiers in Microbiology, 7, 1027.
Bornancin, L., Bonnard, I., Mills, S. C., & Banaigs, B. (2017). Chemical mediation as a structuring element in marine gastropod predator-prey interactions. Natural Product Reports, 34, 644–676.
Boury-Esnault, N., Lavrov, D. V., Ruiz, C. A., & Pérez, T. (2013). The integrative taxonomic approach applied to porifera: A case study of the homoscleromorpha. Integrative and Comparative Biology, 53, 416–427.
Butler, A. J., Van Altena, I. A., & Dunne, S. J. (1996). Antifouling activity oflyso-platelet-activating factor extracted from australian sponge Crella incrustans. Journal of Chemical Ecology, 22, 2041–2061.
Casapullo, A., Minale, L., & Zollo, F. (1993). Paniceins and related sesquiterpenoids from the Mediterranean sponge Reniera fulva. Journal of Natural Products, 56, 527–533.
Casapullo, A., Scognamiglio, G., & Cimino, G. (1997). Mucosin: A new bicyclic eicosanoid from the Mediterranean sponge Reniera mucosa. Tetrahedron Letters, 38, 3643–3646.
Cimino, G., & De Stefano, S. (1977). New acetylenic compounds from the sponge Reniera fulva. Tetrahedron Letters, 18, 1325–1328.
Costa-Lotufo, L. V., Carnevale-Neto, F., Trindade-Silva, A. E., Silva, R. R., Silva, G. G. Z., Wilke, D. V., Pinto, F. C. L., Sahm, B. D. B., Jimenez, P. C., Mendonca, J. N., Lotufo, T. M. C., Pessoa, O. D. L., & Lopes, N. P. (2018). Chemical profiling of two congeneric sea mat corals along the Brazilian coast: Adaptive and functional patterns. Chemical Communications, 54, 1952–1955.
De Caralt, S., Bry, D., Bontemps, N., Turon, X., Uriz, M.-J., & Banaigs, B. (2013). Sources of secondary metabolite variation in dysidea avara (Porifera: Demospongiae): The importance of having good neighbors. Marine Drugs, 11, 489.
De Goeij, J. M., Van Oevelen, D., Vermeij, M. J. A., Osinga, R., Middelburg, J. J., De Goeij, A. F. P. M., & Admiraal, W. (2013). Surviving in a marine desert: The sponge loop retains resources within coral reefs. Science, 342, 108–110.
De’ath, G. (2002). Multivariate regression trees: A new technique for modeling species-environment relationships. Ecology, 83, 1105–1117.
Dembitsky, V. M., Rezanka, T., & Srebnik, M. (2003). Lipid compounds of freshwater sponges: Family Spongillidae, class Demospongiae. Chemistry and Physics of Lipids, 123, 117–155.
Duckworth, A. R., West, L., Vansach, T., Stubler, A., & Hardt, M. (2012). Effects of water temperature and pH on growth and metabolite biosynthesis of coral reef sponges. Marine Ecology Progress Series, 462, 67–77.
Ereskovsky, A. V., Geronimo, A., & Pérez, T. (2017). Asexual and puzzling sexual reproduction of the Mediterranean sponge Haliclona fulva (Demospongiae): Life cycle and cytological structures. Invertebrate Biology, 136, 403–421.
Erpenbeck, D., & Van Soest, R. W. M. (2006). Status and perspective of sponge chemosystematics. Marine Biotechnology, 9, 2.
Ferrer, R. P., & Zimmer, R. K. (2012). Community ecology and the evolution of molecules of keystone significance. The Biological Bulletin, 223, 167–177.
Ferrer, R. P., & Zimmer, R. K. (2013). Molecules of keystone significancecrucial agents in ecology and resource management. BioScience, 63, 428–438.
Genta-Jouve, G., & Thomas, O. P. (2013). Absolute configuration of the New 3-epi-cladocroic acid from the Mediterranean sponge Haliclona Fulva. Metabolites, 3, 24–31.
Glassmire, A. E., Jeffrey, C. S., Forister, M. L., Parchman, T. L., Nice, C. C., Jahner, J. P., Wilson, J. S., Walla, T. R., Richards, L. A., Smilanich, A. M., Leonard, M. D., Morrison, C. R., Simbaña, W., Salagaje, L. A., Dodson, C. D., Miller, J. S., Tepe, E. J., Villamarin-Cortez, S., & Dyer, L. A. (2016). Intraspecific phytochemical variation shapes community and population structure for specialist caterpillars. New Phytologist, 212, 208–219.
Goulitquer, S., Potin, P., & Tonon, T. (2012). Mass spectrometry-based metabolomics to elucidate functions in marine organisms and ecosystems. Marine Drugs, 10, 849–880.
Haas, A. F., Fairoz, M. F. M., Kelly, L. W., Nelson, C. E., Dinsdale, E. A., Edwards, R. A., Giles, S., Hatay, M., Hisakawa, N., Knowles, B., Lim, Y. W., Maughan, H., Pantos, O., Roach, T. N. F., Sanchez, S. E., Silveira, C. B., Sandin, S., Smith, J. E., & Rohwer, F. (2016). Global microbialization of coral reefs. Nature Microbiology, 1, 16042.
Hay, M. E. (2009). Marine chemical ecology: Chemical signals and cues structure marine populations, communities, and ecosystems. Annual Review of Marine Science, 1, 193–212.
Hay, M. E. (2014). Challenges and opportunities in marine chemical ecology. Journal of Chemical Ecology, 40, 216–217.
He, Q., Sun, R., Liu, H., Geng, Z., Chen, D., Li, Y., Han, J., Lin, W., Du, S., & Deng, Z. (2014). NMR-Based Metabolomic Analysis of Spatial Variation in Soft Corals. Marine Drugs, 12, 1876–1890.
Ivanisevic, J., Thomas, O. P., Pedel, L., Pénez, N., Ereskovsky, A. V., Culioli, G., & Pérez, T. (2011). Biochemical trade-offs: Evidence for ecologically linked secondary metabolism of the sponge Oscarella balibaloi. PLoS ONE, 6, e28059.
Ivanišević, J., Perez, T., Ereskovsky, A. V., Barnathan, G., & Thomas, O. P. (2011). Lysophospholipids in the Mediterranean sponge Oscarella tuberculata: Seasonal variability and putative biological role. Journal of Chemical Ecology, 37, 537–545.
Kelman, D., Benayahu, Y., & Kashman, Y. (2000). Variation in secondary metabolite concentrations in yellow and grey morphs of the red sea soft coral Parerythropodium fulvum fulvum: Possible ecological implications. Journal of Chemical Ecology, 26, 1123–1133.
Kessner, D., Chambers, M., Burke, R., Agus, D., & Mallick, P. (2008). ProteoWizard: Open source software for rapid proteomics tools development. Bioinformatics, 24, 2534–2536.
Kosmides, A. K., Kamisoglu, K., Calvano, S. E., Corbett, S. A., & Androulakis, I. P. (2013). Metabolomic fingerprinting: Challenges and opportunities. Critical Reviews in Biomedical Engineering, 41, 205–221.
Kuhlisch, C., & Pohnert, G. (2015). Metabolomics in chemical ecology. Natural Product Reports, 32, 937–955.
Li, D., Baldwin, I. T., & Gaquerel, E. 2015. Navigating natural variation in herbivory-induced secondary metabolism in coyote tobacco populations using MS/MS structural analysis. Proceedings of the National Academy of Sciences, 112, E4147–E4155.
Lin, K., Yang, P., Yang, H., Liu, A.-H., Yao, L.-G., Guo, Y.-W., & Mao, S.-C. (2015). Lysophospholipids from the Guangxi sponge Spirastrella purpurea. Lipids, 50, 697–703.
Loh, T.-L., & Pawlik, J. R. (2014). Chemical defenses and resource trade-offs structure sponge communities on Caribbean coral reefs. Proceedings of the National Academy of Sciences of the United States of America, 111, 4151–4156.
López-Legentil, S., Bontemps-Subielos, N., Turon, X., & Banaigs, B. (2006). Temporal variation in the production of four secondary metabolites in a colonial ascidian. Journal of Chemical Ecology, 32, 2079–2084.
López-Legentil, S., Bontemps-Subielos, N., Turon, X., & Banaigs, B. (2007). Secondary metabolite and inorganic contents in Cystodytes sp. (Ascidiacea): Temporal patterns and association with reproduction and growth. Marine Biology, 151, 293–299.
Moore, B. D., Andrew, R. L., Külheim, C., & Foley, W. J. (2014). Explaining intraspecific diversity in plant secondary metabolites in an ecological context. New Phytologist, 201, 733–750.
Müller, W. E. G., Klemt, M., Thakur, N. L., Schröder, H. C., Aiello, A., D’esposito, M., Menna, M., & Fattorusso, E. (2004). Molecular/chemical ecology in sponges: Evidence for an adaptive antibacterial response in Suberites domuncula. Marine Biology, 144, 19–29.
Nishikawa, Y., Furukawa, A., Shiga, I., Muroi, Y., Ishii, T., Hongo, Y., Takahashi, S., Sugawara, T., Koshino, H., & Ohnishi, M. (2015). Cytoprotective effects of lysophospholipids from sea cucumber Holothuria atra. PLoS ONE, 10, e0135701.
Noyer, C., & Becerro, M. A. (2012). Relationship between genetic, chemical, and bacterial diversity in the Atlanto-Mediterranean bath sponge Spongia lamella. Hydrobiologia, 687, 85–99.
Noyer, C., Thomas, O. P., & Becerro, M. A. (2011). Patterns of chemical diversity in the Mediterranean sponge Spongia lamella. PLoS ONE, 6, e20844.
Nuzzo, G., Ciavatta, M. L., Villani, G., Manzo, E., Zanfardino, A., Varcamonti, M., & Gavagnin, M. (2012). Fulvynes, antimicrobial polyoxygenated acetylenes from the Mediterranean sponge Haliclona fulva. Tetrahedron, 68, 754–760.
Ohta, S., Ogawa, T., Ohta, E., Ikeuchi, T., Kamemura, K., & Ikegami, S. (2013). Petroacetylene, a new polyacetylene from the marine sponge Petrosiasolida that inhibits blastulation of starfish embryos. Natural Product Research, 27, 1842–1847.
Ortega, M. J., Zubía, E., Carballo, J. L., & Salvá, J. (1996). Fulvinol, a new long-chain diacetylenic metabolite from the sponge Reniera fulva. Journal of Natural Products, 59, 1069–1071.
Page, M., West, L., Northcote, P., Battershill, C., & Kelly, M. (2005). Spatial and temporal variability of cytotoxic metabolites in populations of the New Zealand sponge Mycale hentscheli. Journal of Chemical Ecology, 31, 1161–1174.
Patti, G. J., Tautenhahn, R., & Siuzdak, G. (2012). Meta-analysis of untargeted metabolomic data: Combining results from multiple profiling experiments. Nature Protocols, 7, 508–516.
Paul, V. J., Arthur, K. E., Ritson-Williams, R., Ross, C., & Sharp, K. (2007). Chemical defenses: From compounds to communities. The Biological Bulletin, 213, 226–251.
Paul, V. J., & Van Alstyne, K. L. (1992). Activation of chemical defenses in the tropical green algae Halimeda spp. Journal of Experimental Marine Biology and Ecology, 160, 191–203.
Payo, D. A., Colo, J., Calumpong, H., & De Clerck, O. (2011). Variability of non-polar secondary metabolites in the red alga Portieria. Marine Drugs, 9, 2438–2468.
Peters, L., Wright, A. D., Krick, A., & König, G. M. (2004). Variation of brominated indoles and terpenoids within single and different colonies of the marine bryozoan Flustra foliacea. Journal of Chemical Ecology, 30, 1165–1181.
Pham, N. B., Butler, M. S., Hooper, J. N. A., Moni, R. W., & Quinn, R. J. (1999). Isolation of xestosterol esters of brominated acetylenic fatty acids from the marine sponge Xestospongia testudinaria. Journal of Natural Products, 62, 1439–1442.
Pohnert, G. (2004). Chemical defense strategies of marine organisms. In S. SCHULZ (Ed.), The chemistry of pheromones and other semiochemicals I. Berlin: Springer.
Proksch, P. (1994). Defensive roles for secondary metabolites from marine sponges and sponge-feeding nudibranchs. Toxicon, 32, 639–655.
Redmond, N. E., Raleigh, J., Van Soest, R. W. M., Kelly, M., Travers, S. A. A., Bradshaw, B., Vartia, S., Stephens, K. M., & Mccormack, G. P. (2011). Phylogenetic relationships of the marine Haplosclerida (Phylum Porifera) employing ribosomal (28S rRNA) and mitochondrial (cox1, nad1) gene sequence data. PLoS ONE, 6, e24344.
Reverter, M., Perez, T., Ereskovsky, A. V., & Banaigs, B. (2016). Secondary metabolome variability and inducible chemical defenses in the Mediterranean sponge Aplysina cavernicola. Journal of Chemical Ecology, 42, 60–70.
Rhoades, D. F. (1985). Offensive-defensive interactions between herbivores and plants: Their relevance in herbivore population dynamics and ecological theory. The American Naturalist, 125, 205–238.
Routaboul, J.-M., Dubos, C., Beck, G., Marquis, C., Bidzinski, P., Loudet, O., & Lepiniec, L. (2012). Metabolite profiling and quantitative genetics of natural variation for flavonoids in Arabidopsis. Journal of Experimental Botany, 63, 3749–3764.
Sacristan-Soriano, O., Banaigs, B., & Becerro, M. A. (2011). Relevant spatial scales of chemical variation in Aplysina aerophoba. Marine Drugs, 9, 2499–2513.
Sacristán-Soriano, O., Banaigs, B., & Becerro, M. A. (2012). Temporal trends in the secondary metabolite production of the sponge Aplysina aerophoba. Marine Drugs, 10, 677–693.
Santín, A., Grinyó, J., Ambroso, S., Uriz, M. J., Gori, A., Dominguez-Carrió, C., & Gili, J.-M. (2017). Sponge assemblages on the deep Mediterranean continental shelf and slope (Menorca Channel, Western Mediterranean Sea). Deep Sea Research Part I, 131, 75–86.
Schlick-Steiner, B. C., Steiner, F. M., Seifert, B., Stauffer, C., Christian, E., & Crozier, R. H. (2009). Integrative taxonomy: A multisource approach to exploring biodiversity. Annual Review of Entomology, 55, 421–438.
Schmitt, S., Tsai, P., Bell, J., Fromont, J., Ilan, M., Lindquist, N., Perez, T., Rodrigo, A., SCHUPP, P. J., Vacelet, J., Webster, N., Hentschel, U., & Taylor, M. W. (2012). Assessing the complex sponge microbiota: Core, variable and species-specific bacterial communities in marine sponges. The ISME Journal, 6, 564–576.
Shin, B. A., Kim, Y. R., Lee, I.-S., Sung, C. K., Hong, J., Sim, C. J., Im, K. S., & Jung, J. H. (1999). Lyso-PAF analogues and lysophosphatidylcholines from the marine sponge Spirastrella abata as inhibitors of cholesterol biosynthesis. Journal of Natural Products, 62, 1554–1557.
Soares, A. R., Duarte, H. M., Tinnoco, L. W., Pereira, R. C., & Teixeira, V. L. (2015). Intraspecific variation of meroditerpenoids in the brown alga Stypopodium zonale guiding the isolation of new compounds. Revista Brasileira de Farmacognosia, 25, 627–633.
Stanley, D. W. 2000. Eicosanoids in invertebrate signal transduction systems, Princeton: Princeton University Press.
Sugiura, T., Fukuda, T., Miyamoto, T., & Waku, K. (1992). Distribution of alkyl and alkenyl ether-linked phospholipids and platelet-activating factor-like lipid in various species of invertebrates. Biochimica et Biophysica Acta (BBA), 1126, 298–308.
Ternon, E., Perino, E., Manconi, R., Pronzato, R., & THOMAS, O. P. (2017). How environmental factors affect the production of guanidine alkaloids by the Mediterranean sponge Crambe crambe. Marine Drugs, 15, 181.
Tribalat, M.-A. 2016. Specialized metabolisms of Mediterranean sponges of genus Haliclona Grant, 1836. Nice: Université Côte d’Azur.
Tribalat, M.-A., Marra, M. V., Mccormack, G. P., & Thomas, O. P. (2016). Does the chemical diversity of the order Haplosclerida (Phylum Porifera: Class Demospongia) fit with current taxonomic classification? Planta Medica, 82, 843–856.
Vrablik, T. L., & Watts, J. L. (2013). Polyunsaturated fatty acid derived signaling in reproduction and development: Insights from Caenorhabditis elegans and Drosophila melanogaster. Molecular Reproduction and Development, 80, 244–259.
Zhao, Q., Mansoor, T. A., Hong, J., Lee, C.-O., Im, K. S., Lee, D. S., & Jung, J. H. (2003). New lysophosphatidylcholines and monoglycerides from the marine sponge Stelletta sp. Journal of Natural Products, 66, 725–728.
Zubía, E., Ortega, J., Luis Carballo, M., J. & Salvá, J. (1994). Sesquiterpene hydroquinones from the sponge Reniera mucosa. Tetrahedron, 50, 8153–8160.
Acknowledgements
This project (Grant-Aid Agreement No. PBA/MB/16/01) is carried out with the support of the Marine Institute and is funded under the Marine Research Programme by the Irish Government. M.-A.T. received a Ph.D. scholarship from the French Ministry for Higher education and Research. Metabolomic analyses were performed on the MALLABAR platform (Funded by the CNRS, the Provence Alpes Côte d’Azur Region and the Total Foundation). S. Greff (IMBE Marseille, France) is acknowledged for his help in recording and analysing the metabolomic data.
Funding
This project (Grant-Aid Agreement No. PBA/MB/16/01) is carried out with the support of the Marine Institute and is funded under the Marine Research Programme by the Irish Government. The Ph.D. scholarship of M.-A. Tribalat has been funded by the French “Ministère de lʼEnseignement supérieur, de la Recherche et de lʼInnovation”.
Author information
Authors and Affiliations
Contributions
Methodology and Formal Analysis, M.-A.T., T.P., M.R.; Validation, O.P.T.; Writing—Original Draft Preparation, M.R.; Writing—Review & Editing, O.P.T.; Supervision, T.P., O.P.T.; Project Administration, O.P.T.; Funding Acquisition, T.P., O.P.T.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. This article does not contain any studies with human participants performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Reverter, M., Tribalat, MA., Pérez, T. et al. Metabolome variability for two Mediterranean sponge species of the genus Haliclona: specificity, time, and space. Metabolomics 14, 114 (2018). https://doi.org/10.1007/s11306-018-1401-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11306-018-1401-5