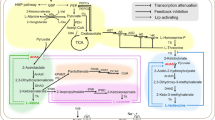
Abstract
l-Amino acids find various applications in biotechnology. l-Glutamic acid and its salts are used as flavor enhancers. Other l-amino acids are used as food or feed additives, in parenteral nutrition or as building blocks for the chemical and pharmaceutical industries. l-amino acids are synthesized from precursors of central carbon metabolism. Based on the knowledge of the biochemical pathways microbial fermentation processes of food, feed and pharma amino acids have been developed. Production strains of Corynebacterium glutamicum, which has been used safely for more than 50 years in food biotechnology, and Escherichia coli are constantly improved using metabolic engineering approaches. Research towards new processes is ongoing. Fermentative production of l-amino acids in the million-ton-scale has shaped modern biotechnology and its markets continue to grow steadily. This review focusses on recent achievements in strain development for amino acid production including the use of CRISPRi/dCas9, genome-reduced strains, biosensors and synthetic pathways to enable utilization of alternative carbon sources.


Similar content being viewed by others
References
Adachi N, Takahashi C, Ono-Murota N, Yamaguchi R, Tanaka T, Kondo A (2013) Direct l-lysine production from cellobiose by Corynebacterium glutamicum displaying beta-glucosidase on its cell surface. Appl Microbiol Biotechnol 97:7165–7172. doi:10.1007/s00253-013-5009-4
Adham SA, Honrubia P, Diaz M, Fernandez-Abalos JM, Santamaria RI, Gil JA (2001) Expression of the genes coding for the xylanase Xys1 and the cellulase Cel1 from the straw-decomposing Streptomyces halstedii JM8 cloned into the amino-acid producer Brevibacterium lactofermentum ATCC13869. Arch Microbiol 177:91–97. doi:10.1007/s00203-001-0365-3
Anwar Z, Gulfraz M, Irshad M (2014) Agro-industrial lignocellulosic biomass a key to unlock the future bio-energy: a brief review. J Radiat Res Appl Sci 7:163–173
Aristidou A, Penttila M (2000) Metabolic engineering applications to renewable resource utilization. Curr Opin Biotechnol 11:187–198
Barrett E, Stanton C, Zelder O, Fitzgerald G, Ross RP (2004) Heterologous expression of lactose- and galactose-utilizing pathways from lactic acid bacteria in Corynebacterium glutamicum for production of lysine in whey. Appl Environ Microbiol 70:2861–2866
Baumgart M, Unthan S, Ruckert C, Sivalingam J, Grunberger A, Kalinowski J, Bott M, Noack S, Frunzke J (2013) Construction of a prophage-free variant of Corynebacterium glutamicum ATCC 13032 for use as a platform strain for basic research and industrial biotechnology. Appl Environ Microbiol 79:6006–6015. doi:10.1128/AEM.01634-13
Bellmann A, Vrljic M, Patek M, Sahm H, Kramer R, Eggeling L (2001) Expression control and specificity of the basic amino acid exporter LysE of Corynebacterium glutamicum. Microbiology 147:1765–1774. doi:10.1099/00221287-147-7-1765
Binder S, Schendzielorz G, Stabler N, Krumbach K, Hoffmann K, Bott M, Eggeling L (2012) A high-throughput approach to identify genomic variants of bacterial metabolite producers at the single-cell level. Genome Biol 13:R40. doi:10.1186/gb-2012-13-5-r40
Binder S, Siedler S, Marienhagen J, Bott M, Eggeling L (2013) Recombineering in Corynebacterium glutamicum combined with optical nanosensors: a general strategy for fast producer strain generation. Nucleic Acids Res 41:6360–6369. doi:10.1093/nar/gkt312
Blombach B, Seibold GM (2010) Carbohydrate metabolism in Corynebacterium glutamicum and applications for the metabolic engineering of l-lysine production strains. Appl Microbiol Biotechnol 86:1313–1322. doi:10.1007/s00253-010-2537-z
Buschke N, Schroder H, Wittmann C (2011) Metabolic engineering of Corynebacterium glutamicum for production of 1,5-diaminopentane from hemicellulose. Biotechnol J 6:306–317. doi:10.1002/biot.201000304
Choi JW, Yim SS, Lee SH, Kang TJ, Park SJ, Jeong KJ (2015) Enhanced production of gamma-aminobutyrate (GABA) in recombinant Corynebacterium glutamicum by expressing glutamate decarboxylase active in expanded pH range. Microb Cell Fact 14:21. doi:10.1186/s12934-015-0205-9
Cleto S, Jensen JV, Wendisch VF, Lu TK (2016) Corynebacterium glutamicum metabolic engineering with CRISPR interference (CRISPRi). ACS Synth Biol. doi:10.1021/acssynbio.5b00216
de Lorenzo V (2015) Chassis organism from Corynebacterium glutamicum: the way towards biotechnological domestication of Corynebacteria. Biotechnol J 10:244–245. doi:10.1002/biot.201400493
Eberhardt D, Jensen JV, Wendisch VF (2014) l-citrulline production by metabolically engineered Corynebacterium glutamicum from glucose and alternative carbon sources. AMB Express 4:85. doi:10.1186/s13568-014-0085-0
Eggeling L, Bott M (2005) Handbook of Corynebacterium glutamicum. CRC, Boca Raton
Eikmanns BJ, Thum-Schmitz N, Eggeling L, Ludtke KU, Sahm H (1994) Nucleotide sequence, expression and transcriptional analysis of the Corynebacterium glutamicum gltA gene encoding citrate synthase. Microbiology 140(Pt 8):1817–1828. doi:10.1099/13500872-140-8-1817
Feher T, Papp B, Pal C, Posfai G (2007) Systematic genome reductions: theoretical and experimental approaches. Chem Rev 107:3498–3513. doi:10.1021/cr0683111
Flynn NE, Meininger CJ, Haynes TE, Wu G (2002) The metabolic basis of arginine nutrition and pharmacotherapy. Biomed Pharmacother 56:427–438
Gerstmeir R, Wendisch VF, Schnicke S, Ruan H, Farwick M, Reinscheid D, Eikmanns BJ (2003) Acetate metabolism and its regulation in Corynebacterium glutamicum. J Biotechnol 104:99–122
Gopinath V, Meiswinkel TM, Wendisch VF, Nampoothiri KM (2011) Amino acid production from rice straw and wheat bran hydrolysates by recombinant pentose-utilizing Corynebacterium glutamicum. Appl Microbiol Biotechnol 92:985–996. doi:10.1007/s00253-011-3478-x
Hao N, Mu J, Hu N, Xu S, Yan M, Li Y, Guo K, Xu L (2015) Improvement of l-citrulline production in Corynebacterium glutamicum by ornithine acetyltransferase. J Ind Microbiol Biotechnol 42:307–313. doi:10.1007/s10295-014-1561-x
Hashimoto M, Ichimura T, Mizoguchi H, Tanaka K, Fujimitsu K, Keyamura K, Ote T, Yamakawa T, Yamazaki Y, Mori H, Katayama T, Kato J (2005) Cell size and nucleoid organization of engineered Escherichia coli cells with a reduced genome. Mol Microbiol 55:137–149. doi:10.1111/j.1365-2958.2004.04386.x
Heider SA, Wolf N, Hofemeier A, Peters-Wendisch P, Wendisch VF (2014) Optimization of the IPP precursor supply for the production of lycopene, decaprenoxanthin and astaxanthin by Corynebacterium glutamicum. Front Bioeng Biotechnol 2:28. doi:10.3389/fbioe.2014.00028
Hii SL, Tan JS, Ling TC, Ariff AB (2012) Pullulanase: role in starch hydrolysis and potential industrial applications. Enzyme Res 2012:921362. doi:10.1155/2012/921362
Hwang JH, Hwang GH, Cho JY (2008) Effect of increased glutamate availability on l-ornithine production in Corynebacterium glutamicum. J Microbiol Biotechnol 18:704–710
Hyeon JE, Jeon WJ, Whang SY, Han SO (2011) Production of minicellulosomes for the enhanced hydrolysis of cellulosic substrates by recombinant Corynebacterium glutamicum. Enzyme Microb Technol 48:371–377. doi:10.1016/j.enzmictec.2010.12.014
Ikeda M, Mitsuhashi S, Tanaka K, Hayashi M (2009) Reengineering of a Corynebacterium glutamicum l-arginine and l-citrulline producer. Appl Environ Microbiol 75:1635–1641. doi:10.1128/AEM.02027-08
Jensen JV, Wendisch VF (2013) Ornithine cyclodeaminase-based proline production by Corynebacterium glutamicum. Microb Cell Fact 12:63. doi:10.1186/1475-2859-12-63
Jensen JV, Eberhardt D, Wendisch VF (2015) Modular pathway engineering of Corynebacterium glutamicum for production of the glutamate-derived compounds ornithine, proline, putrescine, citrulline, and arginine. J Biotechnol 214:85–94. doi:10.1016/j.jbiotec.2015.09.017
Kawaguchi H, Vertes AA, Okino S, Inui M, Yukawa H (2006) Engineering of a xylose metabolic pathway in Corynebacterium glutamicum. Appl Environ Microbiol 72:3418–3428. doi:10.1128/AEM.72.5.3418-3428.2006
Kawaguchi H, Sasaki M, Vertes AA, Inui M, Yukawa H (2008) Engineering of an l-arabinose metabolic pathway in Corynebacterium glutamicum. Appl Microbiol Biotechnol 77:1053–1062. doi:10.1007/s00253-007-1244-x
Kawasaki N, Nakayama A, Yamano N, Takeda S, Kawata Y, Yamamoto N, Aiba S (2005) Synthesis, thermal and mechanical properties and biodegradation of branched polyamide 4. Polymer 46:9987–9993. doi:10.1016/j.polymer.2005.06.092
Kim DJ, Hwang GH, Um JN, Cho JY (2015a) Increased l-ornithine production in Corynebacterium glutamicum by overexpression of a gene encoding a putative aminotransferase. J Mol Microbiol Biotechnol 25:45–50. doi:10.1159/000375124
Kim EM, Um Y, Bott M, Woo HM (2015b) Engineering of Corynebacterium glutamicum for growth and succinate production from levoglucosan, a pyrolytic sugar substrate. FEMS Microbiol Lett. doi:10.1093/femsle/fnv161
Kim SY, Lee J, Lee SY (2015c) Metabolic engineering of Corynebacterium glutamicum for the production of l-ornithine. Biotechnol Bioeng 112:416–421. doi:10.1002/bit.25440
Kinoshita S, Udaka S, Shimono M (2004) Studies on the amino acid fermentation. Part 1. Production of l-glutamic acid by various microorganisms. J Gen Appl Microbiol 50:331–343
Kortmann M, Kuhl V, Klaffl S, Bott M (2015) A chromosomally encoded T7 RNA polymerase-dependent gene expression system for Corynebacterium glutamicum: construction and comparative evaluation at the single-cell level. Microb Biotechnol 8:253–265. doi:10.1111/1751-7915.12236
Kotrba P, Inui M, Yukawa H (2003) A single V317A or V317M substitution in Enzyme II of a newly identified beta-glucoside phosphotransferase and utilization system of Corynebacterium glutamicum R extends its specificity towards cellobiose. Microbiology 149:1569–1580. doi:10.1099/mic.0.26053-0
Lambertz C, Garvey M, Klinger J, Heesel D, Klose H, Fischer R, Commandeur U (2014) Challenges and advances in the heterologous expression of cellulolytic enzymes: a review. Biotechnol Biofuels 7:135. doi:10.1186/s13068-014-0135-5
Lange C, Mustafi N, Frunzke J, Kennerknecht N, Wessel M, Bott M, Wendisch VF (2012) Lrp of Corynebacterium glutamicum controls expression of the brnFE operon encoding the export system for l-methionine and branched-chain amino acids. J Biotechnol 158:231–241. doi:10.1016/j.jbiotec.2011.06.003
Lee SY, Le TH, Chang ST, Park JS, Kim YH, Min J (2010) Utilization of phenol and naphthalene affects synthesis of various amino acids in Corynebacterium glutamicum. Curr Microbiol 61:596–600. doi:10.1007/s00284-010-9658-6
Lee J, Saddler JN, Um Y, Woo HM (2016) Adaptive evolution and metabolic engineering of a cellobiose- and xylose- negative Corynebacterium glutamicum that co-utilizes cellobiose and xylose. Microb Cell Fact 15:20. doi:10.1186/s12934-016-0420-z
Leprince A, de Lorenzo V, Voller P, van Passel MW, Martins dos Santos VA (2012) Random and cyclical deletion of large DNA segments in the genome of Pseudomonas putida. Environ Microbiol 14:1444–1453. doi:10.1111/j.1462-2920.2012.02730.x
Mampel J, Buescher JM, Meurer G, Eck J (2013) Coping with complexity in metabolic engineering. Trends Biotechnol 31:52–60. doi:10.1016/j.tibtech.2012.10.010
Manabe K, Kageyama Y, Morimoto T, Ozawa T, Sawada K, Endo K, Tohata M, Ara K, Ozaki K, Ogasawara N (2011) Combined effect of improved cell yield and increased specific productivity enhances recombinant enzyme production in genome-reduced Bacillus subtilis strain MGB874. Appl Environ Microbiol 77:8370–8381. doi:10.1128/AEM.06136-11
Matano C, Uhde A, Youn JW, Maeda T, Clermont L, Marin K, Kramer R, Wendisch VF, Seibold GM (2014) Engineering of Corynebacterium glutamicum for growth and l-lysine and lycopene production from N-acetyl-glucosamine. Appl Microbiol Biotechnol 98:5633–5643. doi:10.1007/s00253-014-5676-9
Matsui D, Oikawa T (2010) Detection of d-ornithine extracellularly produced by Corynebacterium glutamicum ATCC 13032:argF. Biosci Biotechnol Biochem 74:2507–2510
Meiswinkel TM, Gopinath V, Lindner SN, Nampoothiri KM, Wendisch VF (2013) Accelerated pentose utilization by Corynebacterium glutamicum for accelerated production of lysine, glutamate, ornithine and putrescine. Microb Biotechnol 6:131–140. doi:10.1111/1751-7915.12001
Merkens H, Beckers G, Wirtz A, Burkovski A (2005) Vanillate metabolism in Corynebacterium glutamicum. Curr Microbiol 51:59–65. doi:10.1007/s00284-005-4531-8
Mustafi N, Grunberger A, Kohlheyer D, Bott M, Frunzke J (2012) The development and application of a single-cell biosensor for the detection of l-methionine and branched-chain amino acids. Metab Eng 14:449–457. doi:10.1016/j.ymben.2012.02.002
Mustafi N, Grunberger A, Mahr R, Helfrich S, Noh K, Blombach B, Kohlheyer D, Frunzke J (2014) Application of a genetically encoded biosensor for live cell imaging of l-valine production in pyruvate dehydrogenase complex-deficient Corynebacterium glutamicum strains. PLoS One 9:e85731. doi:10.1371/journal.pone.0085731
Okai N, Takahashi C, Hatada K, Ogino C, Kondo A (2014) Disruption of pknG enhances production of gamma-aminobutyric acid by Corynebacterium glutamicum expressing glutamate decarboxylase. AMB Express 4:20. doi:10.1186/s13568-014-0020-4
Paradis FW, Warren RA, Kilburn DG, Miller RC Jr (1987) The expression of Cellulomonas fimi cellulase genes in Brevibacterium lactofermentum. Gene 61:199–206
Park SJ, Kim EY, Noh W, Oh YH, Kim HY, Song BK, Cho KM, Hong SH, Lee SH, Jegal J (2013) Synthesis of nylon 4 from gamma-aminobutyrate (GABA) produced by recombinant Escherichia coli. Bioprocess Biosyst Eng 36:885–892. doi:10.1007/s00449-012-0821-2
Park SH, Kim HU, Kim TY, Park JS, Kim SS, Lee SY (2014) Metabolic engineering of Corynebacterium glutamicum for l-arginine production. Nat Commun 5:4618. doi:10.1038/ncomms5618
Qi SW, Chaudhry MT, Zhang Y, Meng B, Huang Y, Zhao KX, Poetsch A, Jiang CY, Liu S, Liu SJ (2007) Comparative proteomes of Corynebacterium glutamicum grown on aromatic compounds revealed novel proteins involved in aromatic degradation and a clear link between aromatic catabolism and gluconeogenesis via fructose-1,6-bisphosphatase. Proteomics 7:3775–3787. doi:10.1002/pmic.200700481
Qiao Y, Peng Q, Yan J, Wang H, Ding H, Shi B (2015) Gene cloning and enzymatic characterization of alkali-tolerant type I pullulanase from Exiguobacterium acetylicum. Lett Appl Microbiol 60:52–59. doi:10.1111/lam.12333
Rittmann D, Lindner SN, Wendisch VF (2008) Engineering of a glycerol utilization pathway for amino acid production by Corynebacterium glutamicum. Appl Environ Microbiol 74:6216–6222. doi:10.1128/AEM.00963-08
Rohe P, Venkanna D, Kleine B, Freudl R, Oldiges M (2012) An automated workflow for enhancing microbial bioprocess optimization on a novel microbioreactor platform. Microb Cell Fact 11:144. doi:10.1186/1475-2859-11-144
Rumbold K, van Buijsen HJ, Gray VM, van Groenestijn JW, Overkamp KM, Slomp RS, van der Werf MJ, Punt PJ (2010) Microbial renewable feedstock utilization: a substrate-oriented approach. Bioeng Bugs 1:359–366. doi:10.4161/bbug.1.5.12389
Salvatore F, Cimino F, Dayello Caracciolo M, Cittadini D (1964) Mechanism of the protection by l-Ornithine-l-Aspartate mixture and by l-Arginine in ammonia intoxication. Arch Biochem Biophys 107:499–503
Sasaki M, Jojima T, Kawaguchi H, Inui M, Yukawa H (2009) Engineering of pentose transport in Corynebacterium glutamicum to improve simultaneous utilization of mixed sugars. Appl Microbiol Biotechnol 85:105–115. doi:10.1007/s00253-009-2065-x
Schendzielorz G, Dippong M, Grunberger A, Kohlheyer D, Yoshida A, Binder S, Nishiyama C, Nishiyama M, Bott M, Eggeling L (2014) Taking control over control: use of product sensing in single cells to remove flux control at key enzymes in biosynthesis pathways. ACS Synth Biol 3:21–29. doi:10.1021/sb400059y
Schneider J, Niermann K, Wendisch VF (2011) Production of the amino acids l-glutamate, l-lysine, l-ornithine and l-arginine from arabinose by recombinant Corynebacterium glutamicum. J Biotechnol 154:191–198. doi:10.1016/j.jbiotec.2010.07.009
Schwarz WH (2001) The cellulosome and cellulose degradation by anaerobic bacteria. Appl Microbiol Biotechnol 56:634–649
Seibold G, Auchter M, Berens S, Kalinowski J, Eikmanns BJ (2006) Utilization of soluble starch by a recombinant Corynebacterium glutamicum strain: growth and lysine production. J Biotechnol 124:381–391. doi:10.1016/j.jbiotec.2005.12.027
Shen XH, Liu ZP, Liu SJ (2004) Functional identification of the gene locus (ncg12319 and characterization of catechol 1,2-dioxygenase in Corynebacterium glutamicum. Biotechnol Lett 26:575–580
Shen X-H, Huang Y, Liu S-J (2005a) Genomic analysis and identification of catabolic pathways for aromatic compounds in Corynebacterium glutamicum. Microb Environ 20(3):160–167. doi:10.1264/jsme2.20.160
Shen XH, Jiang CY, Huang Y, Liu ZP, Liu SJ (2005b) Functional identification of novel genes involved in the glutathione-independent gentisate pathway in Corynebacterium glutamicum. Appl Environ Microbiol 71:3442–3452. doi:10.1128/AEM.71.7.3442-3452.2005
Shen XH, Zhou NY, Liu SJ (2012) Degradation and assimilation of aromatic compounds by Corynebacterium glutamicum: another potential for applications for this bacterium? Appl Microbiol Biotechnol 95:77–89. doi:10.1007/s00253-012-4139-4
Shi HP, Fishel RS, Efron DT, Williams JZ, Fishel MH, Barbul A (2002) Effect of supplemental ornithine on wound healing. J Surg Res 106:299–302
Shi F, Jiang J, Li Y, Li Y, Xie Y (2013) Enhancement of gamma-aminobutyric acid production in recombinant Corynebacterium glutamicum by co-expressing two glutamate decarboxylase genes from Lactobacillus brevis. J Ind Microbiol Biotechnol 40:1285–1296. doi:10.1007/s10295-013-1316-0
Suzuki N, Nonaka H, Tsuge Y, Inui M, Yukawa H (2005) New multiple-deletion method for the Corynebacterium glutamicum genome, using a mutant lox sequence. Appl Environ Microbiol 71:8472–8480. doi:10.1128/AEM.71.12.8472-8480.2005
Takahashi C, Shirakawa J, Tsuchidate T, Okai N, Hatada K, Nakayama H, Tateno T, Ogino C, Kondo A (2012) Robust production of gamma-amino butyric acid using recombinant Corynebacterium glutamicum expressing glutamate decarboxylase from Escherichia coli. Enzyme Microb Technol 51:171–176. doi:10.1016/j.enzmictec.2012.05.010
Tateno T, Fukuda H, Kondo A (2007) Direct production of l-lysine from raw corn starch by Corynebacterium glutamicum secreting Streptococcus bovis alpha-amylase using cspB promoter and signal sequence. Appl Microbiol Biotechnol 77:533–541. doi:10.1007/s00253-007-1191-6
Trotschel C, Deutenberg D, Bathe B, Burkovski A, Kramer R (2005) Characterization of methionine export in Corynebacterium glutamicum. J Bacteriol 187:3786–3794. doi:10.1128/JB.187.11.3786-3794.2005
Uhde A, Youn JW, Maeda T, Clermont L, Matano C, Kramer R, Wendisch VF, Seibold GM, Marin K (2013) Glucosamine as carbon source for amino acid-producing Corynebacterium glutamicum. Appl Microbiol Biotechnol 97:1679–1687. doi:10.1007/s00253-012-4313-8
Unthan S, Baumgart M, Radek A, Herbst M, Siebert D, Bruhl N, Bartsch A, Bott M, Wiechert W, Marin K, Hans S, Kramer R, Seibold G, Frunzke J, Kalinowski J, Ruckert C, Wendisch VF, Noack S (2015a) Chassis organism from Corynebacterium glutamicum—a top-down approach to identify and delete irrelevant gene clusters. Biotechnol J 10:290–301. doi:10.1002/biot.201400041
Unthan S, Radek A, Wiechert W, Oldiges M, Noack S (2015b) Bioprocess automation on a mini pilot plant enables fast quantitative microbial phenotyping. Microb Cell Fact 14:32. doi:10.1186/s12934-015-0216-6
van Ooyen J, Noack S, Bott M, Reth A, Eggeling L (2012) Improved l-lysine production with Corynebacterium glutamicum and systemic insight into citrate synthase flux and activity. Biotechnol Bioeng 109:2070–2081. doi:10.1002/bit.24486
Vrljic M, Sahm H, Eggeling L (1996) A new type of transporter with a new type of cellular function: l-lysine export from Corynebacterium glutamicum. Mol Microbiol 22:815–826
Wendisch VF (2014) Microbial production of amino acids and derived chemicals: synthetic biology approaches to strain development. Curr Opin Biotechnol 30:51–58. doi:10.1016/j.copbio.2014.05.004
Wyman CE (1999) Biomass ethanol: technical progress, opportunities, and commercial challenges. Ann Rev Energy Environ 24(1):189–226
Zahoor A, Lindner SN, Wendisch VF (2012) Metabolic engineering of Corynebacterium glutamicum aimed at alternative carbon sources and new products. Comput Struct Biotechnol J 3:e201210004. doi:10.5936/csbj.201210004
Zhou LB, Zeng AP (2015a) Engineering a lysine-ON riboswitch for metabolic control of lysine production in Corynebacterium glutamicum. ACS Synth Biol 4:1335–1340. doi:10.1021/acssynbio.5b00075
Zhou LB, Zeng AP (2015b) Exploring lysine riboswitch for metabolic flux control and improvement of l-lysine synthesis in Corynebacterium glutamicum. ACS Synth Biol 4:729–734. doi:10.1021/sb500332c
Funding information
João M.P. Jorge and Fernando Pérez-García are fellows of the CLIB2021 graduate cluster at Bielefeld University. Support by Grant KF2969004SB4 from ZIM, BMWi, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This article does not contain any studies with human participants or animals performed by any of the authors.
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Wendisch, V.F., Jorge, J.M.P., Pérez-García, F. et al. Updates on industrial production of amino acids using Corynebacterium glutamicum . World J Microbiol Biotechnol 32, 105 (2016). https://doi.org/10.1007/s11274-016-2060-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11274-016-2060-1