Abstract
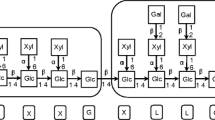
A putative endo-1,4-β-d-xylanohydrolase gene xyl11 from Aspergillus niger, encoding a 188-residue xylanase of glycosyl hydrolase family 11, was constitutively expressed in Pichia pastoris. The recombinant Xyl11 exhibited optimal activity at pH 5.0 and 50 °C, and displayed more than 68 % of the maximum activity over the temperature range 35–65 °C and 33 % over the pH range 2.2–7.0. It maintained more than 40 % of the original activity after incubation at 90 °C (pH 5.0) for 10 min and more than 75 % of the original activity after incubation at pH 2.2–11.0 (room temperature) for 2 h. The specific activity, K m and V max of purified Xyl11 were 22,253 U mg−1, 6.57 mg ml−1 and 51,546.4 μmol min−1 mg−1. It could degrade xylan to a series of xylooligosaccharides and no xylose was detected. The recombinant enzyme with high stability and catalytic efficiency could work over wide ranges of pH and temperature and thus has the potential for various industrial applications.






Similar content being viewed by others
References
Ahlawat S, Battan B, Dhiman SS, Sharma J, Mandhan RP (2007) Production of thermostable pectinase and xylanase for their potential application in bleaching of kraft pulp. J Ind Microbiol Biotechnol 34(12):763–770
Ahmed S, Riaz S, Jamil A (2009) Molecular cloning of fungal xylanases: an overview. Appl Microbiol Biotechnol 84(1):19–35
Bai Y, Wang J, Zhang Z, Yang P, Shi P, Luo H, Meng K, Huang H, Yao B (2010) A new xylanase from thermoacidophilic Alicyclobacillus sp. A4 with broad-range pH activity and pH stability. J Ind Microbiol Biotechnol 37(2):187–194
Bailey MJ, Biely P, Poutanen K (1992) Interlaboratory testing of methods for assay of xylanase activity. J Biotechnol 23(3):257–270
Canakcı S, Cevher Z, Inan K, Tokgoz M, Bahar F, Kacagan M, Sal FA, Belduz AO (2012) Cloning, purification and characterization of an alkali-stable endoxylanase from thermophilic Geobacillus sp. 71. World J Microbiol Biotechnol 28(5):1981–1988
Cao Y, Qiao J, Li Y, Lu W (2007) De novo synthesis, constitutive expression of Aspergillus sulphureus β-xylanase gene in Pichia pastoris and partial enzymic characterization. Appl Microbiol Biotechnol 76(3):579–585
Cavka A, Alriksson B, Rose SH, van Zyl WH, Jönsson LJ (2011) Biorefining of wood: combined production of ethanol and xylanase from waste fiber sludge. J Ind Microbiol Biotechnol 38(8):891–899
Collins T, Gerday C, Feller G (2005) Xylanases, xylanase families and extremophilic xylanases. FEMS Microbiol Rev 29(1):3–23
Cristobal S, Zemla A, Fischer D, Rychlewski L, Elofsson A (2001) A study of quality measures for protein threading models. BMC Bioinforma 2(1):5
de Carvalho Peixoto-Nogueira S, Michelin M, Betini JHA, Jorge JA, Terenzi HF, de Lourdes Teixeira de Moraes Polizeli M (2009) Production of xylanase by Aspergilli using alternative carbon sources: application of the crude extract on cellulose pulp biobleaching. J Ind Microbiol Biotechnol 36(1):149–155
Giridhar PV, Chandra T (2010) Production of novel halo-alkali-thermo-stable xylanase by a newly isolated moderately halophilic and alkali-tolerant Gracilibacillus sp. TSCPVG. Process Biochem 45(10):1730–1737
Kui H, Luo H, Shi P, Bai Y, Yuan T, Wang Y, Yang P, Dong S, Yao B (2010) Gene cloning, expression, and characterization of a thermostable xylanase from Nesterenkonia xinjiangensis CCTCC AA001025. Appl Biochem Biotechnol 162(4):953–965
Luo H, Li J, Yang J, Wang H, Yang Y, Huang H, Shi P, Yuan T, Fan Y, Yao B (2009a) A thermophilic and acid stable family-10 xylanase from the acidophilic fungus Bispora sp. MEY-1. Extremophiles 13(5):849–857
Luo H, Wang Y, Li J, Yang J, Yang Y, Huang H, Fan Y, Yao B (2009b) Cloning, expression and characterization of a novel acidic xylanase, XYL11B, from the acidophilic fungus Bispora sp. MEY-1. Enzyme Microb Technol 45(2):126–133
Masui DC, Zimbardi ALRL, Souza FHM, Guimarães LHS, Furriel RPM, Jorge JA (2012) Production of a xylose-stimulated β-glucosidase and a cellulase-free thermostable xylanase by the thermophilic fungus Humicola brevis var. thermoidea under solid state fermentation. World J Microbiol Biotechnol 28(8):2689–2701
Nagar S, Gupta VK, Kumar D, Kumar L, Kuhad RC (2010) Production and optimization of cellulase-free, alkali-stable xylanase by Bacillus pumilus SV-85S in submerged fermentation. J Ind Microbiol Biotechnol 37(1):71–83
Pel HJ, de Winde JH, Archer DB, Dyer PS, Hofmann G, Schaap PJ, Turner G, de Vries RP, Albang R, Albermann K (2007) Genome sequencing and analysis of the versatile cell factory Aspergillus niger CBS 513.88. Nat Biotechnol 25(2):221–231
Prakash P, Jayalakshmi S, Prakash B, Rubul M, Sreeramulu K (2012) Production of alkaliphilic, halotolerent, thermostable cellulase free xylanase by Bacillus halodurans PPKS-2 using agro waste: single step purification and characterization. World J Microbiol Biotechnol 28(1):183–192
Saleem M, Aslam F, Akhtar MS, Tariq M, Rajoka MI (2012) Characterization of a thermostable and alkaline xylanase from Bacillus sp. and its bleaching impact on wheat straw pulp. World J Microbiol Biotechnol 28(2):513–522
Sandhu G, Aleff R, Kline B (1992) Dual asymmetric PCR: one-step construction of synthetic genes. Biotechniques 12(1):14–16
Schägger H (2006) Tricine-SDS-PAGE. Nature Protoc 1(1):16–22
Simpson HD, Haufler UR, Daniel RM (1991) An extremely thermostable xylanase from the thermophilic eubacterium Thermotoga. Biochem J 277(Pt 2):413–417
Teixeira RSS, Siqueira FG, Souza MV, Filho EXF, Bon EPS (2010) Purification and characterization studies of a thermostable β-xylanase from Aspergillus awamori. J Ind Microbiol Biotechnol 37(10):1041–1051
Uhl AM, Daniel RM (1999) The first description of an archaeal hemicellulase: the xylanase from Thermococcus zilligii strain AN1. Extremophiles 3(4):263–267
Zhang M, Jiang Z, Yang S, Hua C, Li L (2010) Cloning and expression of a Paecilomyces thermophila xylanase gene in E. coli and characterization of the recombinant xylanase. Bioresour Technol 101(2):688–695
Acknowledgments
This work was financially supported by Genencor Innovation Grant.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Guo, N., Zheng, J., Tian, J. et al. Characterization and constitutive expression of an acidic mesophilic endo-1,4-β-d-xylanohydrolase with high thermotolerance and catalytic efficiency in Pichia pastoris . World J Microbiol Biotechnol 29, 2095–2103 (2013). https://doi.org/10.1007/s11274-013-1374-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-013-1374-5