Abstract
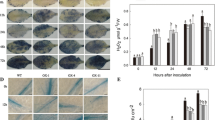
This study aimed to examine the induction of defense responses in tomato elicited by Methylobacterium oryzae CBMB20 as a consequence of reduced stress ethylene level possibly through its ACC deaminase activity. Significantly increased activities of pathogenesis-related (PR) proteins and defense enzymes such as β-1,3-glucanase, phenylalanine ammonia-lyase, peroxidase and polyphenol oxidase were noted in M. oryzae CBMB20 pretreated and challenged with Pseudomonas syringae pv. tomato (Pst) compared to either control or M. oryzae-treated tomato plants in both growth chamber and greenhouse conditions. Increased PR proteins and defense enzyme activities were correlated with the reduction of stress ethylene level. M. oryzae CBMB20 reduced the stress ethylene level about 27% and 55% when challenged with Pst, in growth chamber and greenhouse on day 7 respectively and the effect was comparable to that of the chemical ethylene biosynthesis inhibitor AVG, L-α-(2-aminoethoxyvinyl)-glycine hydrochloride. As a consequence of reduced stress ethylene level and its effect on defense response in crop plants, the disease severity was reduced 26% in M. oryzae CBMB20-treated plants challenged with pathogen. Therefore, inoculation of M. oryzae CBMB20 would induce the defense enzymes and contribute to the enhanced resistance of tomato plants against the pathogen Pst.





Similar content being viewed by others
References
Anandham R, Indiragandhi P, Kim K, Yim W, Madhaiyan M, Saravanan VS, Chung J, Sa TM (2007) Thiosulfate oxidation and mixotrophic growth of Methylobacterium oryzae. Can J Microbiol 53:869–876
Abeles FB, Morgan PW, Saltveit ME Jr. (1992) Ethylene in plant biology, 2nd edn. Academic Press, New York
Bashan Y, de-Bashan LE (2002) Protection of tomato seedlings against infection by Pseudomonas syringae pv. tomato by using the plant growth promoting bacterium Azospirillum brasilense. Appl Environ Microbiol 68:2637–2643
Boller T (1991) Ethylene in pathogenesis and disease resistance. In: Mattoo AK, Suttle JC (eds) The plant hormone ethylene. CRC Press, Boca Raton, FL, pp 293–314
Cameron RK, Zaton K (2004) Intercellular salicylic acid accumulation is important for age-related resistance in Arabidopsis to Pseudomonas syringae. Physiol Mol Plant Pathol 65:197–209
Chen N, Goodwin PH, Hsiang T (2003) The role of ethylene during the infection of Nicotiana tabacum by Colletotrichum destructivum. J Exp Bot 54:2449–2456
Compant S, Duffy B, Nowak J, Clément C, Barka EA (2005) Use of plant growth promoting bacteria for biocontrol of plant diseases: principles, mechanisms of action, and future prospects. Appl Environ Microbiol 71:4951–4959
Davis BD, Mingioli ES (1950) Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol 60:17–28
El-Shora HM (2002) Properties of phenylalanine ammonia-lyase from marrow cotyledons. Plant Sci 162:1–7
Fu JM, Huang BR (2001) Involvement of antioxidants and lipid peroxidation in the adaptation of two cool-season grasses to localized drought stress. Environ Exp Bot 45:105–114
Glick BR, Bashan Y (1997) Genetic manipulation of plant growth promoting bacteria to enhance biocontrol of phytopathogens. Biotechnol Adv 15:353–378
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190:63–68
Hase S, van Pelt JA, van Loon LC, Pieterse CMJ (2003) Colonization of Arabidopsis roots by Pseudomonas fluorescens primes the plant to produce higher levels of ethylene upon pathogen infection. Physiol Mol Plant Pathol 62:219–226
Huang YF, Chen CT, Kao CH (1993) Salicylic acid inhibits the biosynthesis of ethylene in detached rice leaves. Plant Growth Regul 12:79–82
Liang ZC, Hseu RS, Wang HH (1995) Partial purification and characterization of a 1,3-β-D-glucanase from Ganoderma tsugae. J Ind Microbiol 14:5–9
Lidstrom ME (2001) The aerobic methylotrophic bacteria. In: Dworkin M (ed) The prokaryotes. Berlin, Springer-Verlag, pp 223–244
Madhaiyan M, Poonguzhali S, Senthilkumar M, Seshadri S, Chung H, Yang J, Sundaram S, Sa TM (2004) Growth promotion and induction of systemic resistance in rice cultivar Co-47 (Oryza sativa L.) by Methylobacterium spp. Bot Bull Acad Sin 45:315–324
Madhaiyan M, Poonguzhali S, Ryu JH, Sa TM (2006a) Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase containing Methylobacterium fujisawaense. Planta 224:268–278
Madhaiyan M, Suresh Reddy BV, Anandham R, Senthilkumar M, Poonguzhali S, Sundaram SP, Sa TM (2006b) Plant growth-promoting Methylobacterium induces defense responses in groundnut (Arachis hypogaea L.) compared with rot Pathogens. Curr Microbiol 53:270–276
Mayer AM, Harel E, Shaul RB (1965) Assay of catechol oxidase a critical comparison of methods. Phytochemistry 5:783–789
Meyer JM, Abdallah MA (1992) The fluorescent pigment of Pseudomonas fluorescens: biosynthesis, purification and physicochemical properties. J Gen Microbiol 107:319–328
Omer ZS, Tombolini R, Broberg A, Gerhardson B (2004) Indole-3-acetic acid production by pink-pigmented facultative methylotrophic bacteria. Plant Growth Regul 43:93–96
Podile AR, Laxmi VDV (1998) Seed bacterization with Bacillus subtilis AF1 increases phenylalanine ammonia-lyase and reduces the incidence of fusarial wilt in pigeon pea. J Phytopathol 146:255–259
Robison MM, Shah S, Tamot B, Pauls KP, Moffatt BA, Glick BR (2001) Reduced symptoms of Verticillium wilt in transgenic tomato expressing a bacterial ACC deaminase. Mol Plant Pathol 2:135–145
Ryu JH, Madhaiyan M, Poonguzhali S, Yim WJ, Indira Gandhi P, Kim KA, Anandham R, Young JC, Kim KH, Sa TM (2006) Plant growth substances produced by Methylobacterium spp. and their effect on tomato (Lycopersicon esculentum L.) and red pepper (Capsicum annuum L.) growth. J Microbiol Biotechnol 16:1622–1628
SAS Institute Inc. SAS 9.1® (2004) Companion for windows SAS. SAS Institute Inc., Cary, NC
Schwyn B, Neilands JB (1987) Universal chemical assay for the detection and determination of siderophores. Anal Biochem 160:47–56
Silva HS, Aromeiro RS, Macagnan D, Halfeld-Vierira BA, Pereira MCB, Mounteer A (2004) Rhizobacterial induction of systemic resistance in tomato plants: non-specific protection and increase in enzyme activities. Biol Control 29:288–295
Wang C, Knill E, Glick BR, Défago G (2000) Effect of transferring 1-aminocyclopropane-carboxylic acid (ACC) deaminase genes into Pseudomonas fluorescens strain CHA0 and its gacA derivative CHA96 on their growth-promoting and disease-suppressive capacities. Can J Microbiol 46:898–907
Whittenbury R, Davies SL, Wilkinson JF (1970) Enrichment, isolation and some properties of methane-utilizing bacteria. J Gen Microbiol 61:205–218
Zieslin N, Ben-Zaken R (1993) Peroxidase activity and presence of phenolic substances in peduncles of rose flowers. Plant Physiol Biochem 31:333–339
Acknowledgements
The financial support provided by Brain Korea (BK21) through Ph.D. scholarship to P.I is greatly acknowledged. R.A and M.M. are supported by Korea Research Foundation. We acknowledge the partial financial support provided by Rural Development Administration. Critical reading of this paper by the learned referees is thankfully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Indiragandhi, P., Anandham, R., Kim, K. et al. Induction of defense responses in tomato against Pseudomonas syringae pv. tomato by regulating the stress ethylene level with Methylobacterium oryzae CBMB20 containing 1-aminocyclopropane-1-carboxylate deaminase. World J Microbiol Biotechnol 24, 1037–1045 (2008). https://doi.org/10.1007/s11274-007-9572-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9572-7