Abstract
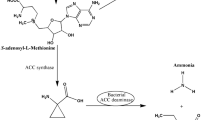
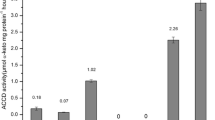
We report the presence of ACC deaminase in Methylobacterium fujisawaense and its lowering of ethylene levels and promotion of root elongation in canola seedlings under gnotobiotic conditions. To test a part of the previous model proposed for ACC deaminase producing bacteria with Methylobacterium, ACC levels and various enzyme activities were monitored in canola. Lower amounts of ACC were present in the tissues of seeds treated with M. fujisawaense strains than in control seeds treated with MgSO4. Though the increased activities of ACC synthase in the tissue extracts of the treated seedlings might be due to bacterial indole-3-acetic acid, the amount of ACC was reduced due to bacterial ACC deaminase activity. The activities of ACC oxidase, the enzyme catalyzing conversion of ACC to ethylene remained lower in M. fujisawaense treated seedlings. This consequently lowered the ethylene in plants and prevented ethylene inhibition of root elongation. Our results collectively suggest that Methylobacterium commonly found in soils, as well as on the surfaces of leaves, seeds, and in the rhizosphere of a wide variety of plants could be better exploited to promote plant growth.




Similar content being viewed by others
Abbreviations
- ACC:
-
1-aminocyclopropane-1-carboxylate
- ACO:
-
ACC oxidase
- ACS:
-
ACC synthase
- AMS:
-
Ammonium mineral salts
- AVG:
-
l-α-(2-aminoethoxyvinyl) glycine hydrochloride
- CFU:
-
Colony-forming units
- IAA:
-
Indole-3-acetic acid
- iPA:
-
Isopentenyladenosine
- MTA:
-
5′-methylthioadenosine
- PGPR:
-
Plant growth promoting rhizobacteria
- PLP:
-
Pyridoxal phosphate
- PPFMs:
-
Pink-pigmented facultative methylotrophic bacteria
- PVPP:
-
Polyvinylpolypyrrolidone
- SAM:
-
S-adenosyl methionine
- t-ZR:
-
trans-Zeatin riboside
References
Abeles FB, Morga PW, Saltveit ME (1992) Ethylene in plant biology. Academic, San Diego
Adams DO, Yang SF (1979) Ethylene biosynthesis: identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci USA 76:170–174
Bayliss C, Bent E, Culham DE, MacLellan S, Clarke AJ, Brown GL, Wood JM (1997) Bacterial genetic loci implicated in the Pseudomonas putida GR12-2R3-canola mutualism: identification of an exudate-inducible sugar transporter. Can J Microbiol 43:809–818
Belimov AA, Safronova VI, Sergeyeva TA, Egorova TN, Matveyeva VA, Tsyganov VE, Borisov AY, Tikhonovich IA, Kluge C, Preisfeld A, Dietz KJ, Stepanok VV (2001) Characterization of plant growth promoting rhizobacteria isolated from polluted soils and containing 1-aminocyclopropane-1-carboxylate deaminase. Can J Microbiol 47:642–652
Bleecker AB, Kende H (2000) Ethylene: a gaseous signal molecule in plants. Annu Rev Cell Dev Biol 16:1–18
Butler HK, Dadson R, Holland MA (2000) Evidence that trans-zeatin riboside produced by a microbial symbiont is physiologically meaningful to its host plant (abstract available at http://www.abstracts.aspb.org/aspp2000/public/P43/0604.html)
Dworkin M, Foster J (1958) Experiments with some microorganisms which utilize ethane and hydrogen. J Bacteriol 75:592–601
Fluhr R, Mattoo AK (1996) Ethylene: biosynthesis and perception. Crit Rev Plant Sci 15:479–523
Freyermuth SK, Long RLG, Mathur S (1996) Metabolic aspects of plant interaction with commensal methylotrophs. In: Lidstrom ME, Tabita FR (eds) Microbial growth on C1 compounds. Kluwer, The Netherlands, pp 277–284
Ghosh S, Penterman JN, Little RD, Chavez R, Glick BR (2003) Three newly isolated plant growth-promoting bacilli facilitate the seedling growth of canola, Brassica campestris. Plant Physiol Biochem 41:277–281
Glick BR (2004) Bacterial ACC deaminase and the alleviation of plant stress. Adv Appl Microbiol 56:291–312
Glick BR, Penrose DM, Li J (1998) A model for the lowering of plant ethylene concentrations by plant growth-promoting bacteria. J Theor Biol 190:63–68
Green PN (1992) The genus Methylobacterium. In: Balows A, Trüper HG, Dworkin M, Harder W, Schleifer KH (eds), The prokaryotes, 2nd edn. vol III. Springer, Berlin Heidelberg New York, pp 2342–2349
Holland MA (1997) Methylobacterium and plants. Rec Res Dev Plant Physiol 1:207–213
Holland MA, Polacco JC (1992) Urease-null and hydrogenase-null phenotypes of a phylloplane bacterium reveal altered nickel metabolism in two soybean mutants. Plant Physiol 98:942–948
Honma M, Shimomura T (1978) Metabolism of 1-aminocyclopropane-1-carboxylic acid. Agric Biol Chem 42:1825–1831
Khalafalla MM, Hattori K (2000) Ethylene inhibitors enhance in vitro root formation on faba bean shoots regenerated on medium containing thidiazuron. Plant Growth Regul 32:59–63
Koenig RL, Morris RO, Polacco JC (2002) tRNA is the source of low-level trans-Zeatin production in Methylobacterium spp. J Bacteriol 184:1832–1842
Lehman A, Black R, Ecker JR (1996) HOOKLESS1, an ethylene response gene, is required for differential cell elongation in the Arabidopsis hypocotyl. Cell 85:183–194
Li J, Ovakim DH, Charles TC, Glick BR (2000) An ACC deaminase minus mutant of Enterobacter cloacae UW4 no longer promotes root elongation. Curr Microbiol 41:101–105
Lizada MCC, Yang SF (1979) A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem 100:142–147
Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with folin-phenol reagent. J Biol Chem 193:265–275
Ma JH, Yao JL, Cohen D, Morris B (1998) Ethylene inhibitors enhance in vitro root formation from apple shoot cultures. Plant Cell Rep 17:211–214
Ma W, Sebestianova SB, Sebestian J, Burd GI, Guinel FC, Glick BR (2003) Prevalence of 1-aminocyclopropane-1-carboxylate deaminase in Rhizobium spp. Anton Leeuw Int J G 83:285–291
Madhaiyan M, Poonguzhali S, Senthilkumar M, Seshadri S, Chung HY, Yang JC, Sundaram SP, Sa TM (2004) Growth promotion and induction of systemic resistance in rice cultivar Co-47 (Oryza sativa L.) by Methylobacterium spp. Bot Bull Acad Sin 45:315–324
Madhaiyan M, Poonguzhali S, Lee HS, Hari K, Sundaram SP, Sa TM (2005a) Pink-pigmented facultative methylotrophic bacteria accelerate germination, growth and yield of sugarcane clone Co86032 (Saccharum officinarum L.). Biol Fertil Soils 41:350–358
Madhaiyan M, Poonguzhali S, Sundaram SP, Sa TM (2005b) A new insight into foliar applied methanol influencing phylloplane methylotrophic dynamics and growth promotion of cotton (Gossypium hirsutum L.) and sugarcane (Saccharum officinarum L.). Environ Exp Bot (published online 15 September 2005)
Malerba M, Crosti P, Armocida D, Bianchetti R (1995) Activation of ethylene production in Acer pseudoplatanus L. cultured cells by fusicoccin. J Plant Physiol 145:93–100
Mattoo AK, Suttle JC (1991) The plant hormone ethylene. CRC Press, Boca Raton, 337 pp
Mayak S, Tirosh T, Glick BR (1999) Effect of wild-type and mutant plant growth-promoting rhizobacteria on the rooting of mung bean cuttings. J Plant Growth Regul 18:49–53
Penrose DM, Glick BR (2001) Levels of ACC and related compounds in exudate and extracts of canola seeds treated with ACC deaminase containing plant growth-promoting bacteria. Can J Microbiol 47:368–372
Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118:10–15
Petruzzelli L, Coraggio I, Leubner-Metzger G (2000) Ethylene promotes ethylene biosynthesis during pea seed germination by positive feedback regulation of 1-aminocyclo-propane-1-carboxylic acid oxidase. Planta 211:144–149
SAS Institute Inc. (2001) SAS user’s guide, version 8.2, SAS Institute Inc., Cary, North Carolina
Satoh S, Oyamada N, Yoshioka T, Midoh N (1997) 1,1-Dimethyl-4-(phenylsulfonyl)semicarbazide (DPSS) does not inhibit the in vitro activities of 1-aminocyclopropane-1-carboxylate (ACC) oxidase and ACC synthase obtained from senescing carnation (Dianthus caryophyllus L.) petals. Plant Growth Regul 23:191–193
Schaller GE, Kieber JJ (2002) Ethylene. The Arabidopsis book. American Society of Plant Biologists, USA
Shah S, Li J, Moffatt BA, Glick BR (1998) Isolation and characterization of ACC deaminase genes from two different plant growth promoting rhizobacteria. Can J Microbiol 44:833–843
Stearns JC, Shah S, Greenberg BM, Dixon DG, Glick BR (2005) Tolerance of transgenic canola expressing 1-aminocyclopropane-1-carboxylic acid deaminase to growth inhibition by nickel. Plant Physiol Biochem 43(7):701–708
Swarup R, Parry G, Graham N, Allen T, Bennett M (2002) Auxin cross-talk: integration of signalling pathways to control plant development. Plant Mol Biol 49:411–426
Trotsenko YA, Ivanova EG, Doronina NV (2001) Aerobic methylotrophic bacteria as phytosymbionts. Microbiology 70:725–736
Wachter R, Fischer K, Gabler R, Kuhnemann F, Urban W, Bogemann GM, Voesenek LACJ, Blom CWPM, Ullrich CI (1999) Ethylene production and ACC accumulation Agrobacterium tumefaciens-induced plant tumours and their impact on tumour and host stem structure and function. Plant Cell Environ 22:1263–1273
Yi HC, Joo S, Nam KH, Lee JS, Kang BG, Kim WT (1999) Auxin and brassinosteroid differentially regulate the expression of three members of the 1-aminocyclopropane-1-carboxylate synthase gene family in mung bean (Vigna radiata L.). Plant Mol Biol 41:443–454
Acknowledgments
This work was supported by grants from the Korea Research Foundation, under the Foreign Scientist and Engineers programme research awarded to M. Madhaiyan and Rural Development Administration (RDA), Republic of Korea. The authors wish to thank anonymous referees for their valuable comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Madhaiyan, M., Poonguzhali, S., Ryu, J. et al. Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase-containing Methylobacterium fujisawaense . Planta 224, 268–278 (2006). https://doi.org/10.1007/s00425-005-0211-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-005-0211-y