Abstract
Expression profiling for genes involved in Vitamin B6 (VitB6) biosynthesis was undertaken to delineate the involvement of de novo and salvage pathway induced by Bacillus subtilis CBR05 against, Xanthomonas campestris pv. vesicatoria in tomato. Pyridoxine biosynthesis (PDX) genes such as PDX1.2 and PDX1.3, were found to be overexpressed significantly at 72 hpi in B. subtilis and pyridoxine inoculated plants. Most significant upregulation was observed in the transcript profile of PDX1.3, which showed more than 12- fold increase in expression. Unfortunately, salt sensitive overlay4 (SOS4) profiling showed irregular expression which corroborates that SOS4 role in VitB6 biosynthesis needs further studies for deciphering a clear notion about their role in tomato. Antioxidant enzymes i.e., superoxide dismutase, catalase, polyphenol oxidase, and peroxidase activities clearly demonstrate escalation till 48 hpi and gets reduced in 72 hpi. Pot trials also confirm that B. subtilis compared to pyridoxine supplementation alone show plant disease resistance and elongated roots. The present study confirms that B. subtilis, as a versatile agent in eliciting induced systemic resistance regulated by de novo pathway as a model for plant defense against X. campestris pv. vesicatoria substantiated by VitB6 biosynthesis. Nevertheless, the study is preliminary and needs further evidence for affirming this phenomenon.
Similar content being viewed by others
Introduction
The cofactor Vitamin (VitB6) is a combination of six water-soluble vital vitamers with a common pyridine ring composed of variations in 4′ moieties possessing alcohol pyridoxine (PN), amine pyridoxamine (PM), aldehyde pyridoxal (PL) and 5′ phosphorylated forms (PNP, PMP, PLP)1. The PLP vitamer plays an essential role as an enzymatic co-factor in more than 140 biochemical reactions and has also recently been implicated in defense against cellular oxidative stress. In plants, two different pathways such as de novo and salvage pathway have been described for biosynthesis of vitB6 vitamers2,3,4,5,6. First, the deoxy-xylulose 5-phosphate (DXP) independent pathway was used for de novo biosynthesis of vitB6 vitamers4. It involves the concerted activities of the pyridoxine biosynthesis proteins (PDX) such as pyridoxine biosynthesis protein1 (PDX1) and 2 (PDX2), that form a multimeric protein complex to synthesize pyridoxal-5′-phosphate (PLP) as an active cofactor2,3,4,5. Second, the salvage pathway predominantly is responsible for the conversion of vitamer forms through specific enzyme modificiation6,7. It converts, PN, PM, and PL to active co-factor PLP by the concerted activities of a vitB6 kinase (salt overlay sensitive 4 (SOS4)) through phosphorylation while biosynthesis of PLP from PN and PM requires the activity of a vitB6 oxidase (PDX3)8,9. Recently, a pyridoxal reductase has been characterized in Arabidopsis thaliana, which is essential for conversion of PL into PN10.
Studies showed that abiotic stress response comprises of pyridoxal kinase and pyridoxal reductase enzymes which play a significant role mediated by salvage pathway8,9,10,11. Previous studies also showed that abiotic stress regulates up-regulation of de novo biosynthetic pathway genes12,13,14,15,16,17. A. thaliana (AtPDX1) gene mutation renders the plants for the high rate of sensitivity to stress conditions like high light, salt and stress. Nevertheless, PDX gene overexpression provides escalated tolerance in combating oxidative stress16,17. The gene for pyridoxal kinase, SOS4 which is a key factor in the salvage pathway was alienated to sequential response to salinity and osmotic stress8,9,10,11.
Moreover, previous studies clearly depict salicylic acid (SA), methyl jasmonate (JA), and ethylene (ET) act as the chemical counterparts in inducing plant defense response abating oxidative stress through overexpression of PDX transcript in Nicotiana tabacum and Hevea brasiliensis18,19. The studies lucidly show that the hypersensitive response (HR) in tobacco leaves infected with Pseudomonas syringae pv. phaseolicola was affected with excess VitB6 content, rather P. syringae pv. tabaci infection resulted in augmented disease severity18. However, direct molecular evidence supporting a role for VitB6 in biocontrol agent, Bacillus subtilis inoculated plants and their plant biotic defense remains lacking. The present study was focused to analyze the expression profiling of de novo (PDX1.2, PDX1.3, PDX2), and salvage pathway (SOS4) genes to demarcate involvement of de novo and salvage pathway in VitB6 biosynthesis by B. subtilis CBR05 upon challenge emancipated by Xanthomonas campestris pv. vesicatoria (XCV) in tomato.
Among plant diseases encountered worldwide, bacterial spot disease, caused by XCV pose a serious threat owing to complex pathogen variability which affects economically important crops production20,21,22,23,24,25. Bacterial diversity poses additional threats to biocontrol efficacy of various agents of antagonistic control and inadequacy of effective strategies to gain disease resistance and efficient abatement through chemical control26. Besides, restrictions on the use of chemical pesticides due to concerns about their impact on the environment and human health are increasing rapidly27. Biological control of disease using microbial antagonists is an eco-friendly alternative to chemical pesticide and is being studied extensively on several different plant diseases28,29,30,31,32. Strains of the Gram-positive bacterium, B. subtilis have been regarded to protect plants opposing fungal and bacterial pathogens. B. subtilis has been regarded as a versatile bacterium possessing plant growth promotion along with enhanced crop protection mediated by ISR (induced systemic resistance)30,31,33. Elicitation of ISR by these strains has demonstrated importance in greenhouse or field trials34,35,36,37,38. Mode of action by which B. subtilis instilling broad-spectrum antagonistic activity against various phytopathogens is attributed to the ability to produce spores, antibiotic production, lytic enzymes and capacity to resists adverse environments39,40,41. Thus, it is clearly evident that Bacillus sp. is an essential regulator of ISR.
B. subtilis is also a predominant endophytic bacterium which marks it as an efficient biocontrol agent against vascular pathogens28,42. Endophyte colonization triggers the reprogramming of the host action, favoring secondary metabolism and inducing changes in the plant development43. For example, B. subtilis strain confers protection to melon plants encountering the cucurbit powdery mildew by activating SA and JA dependent defense response44. Moreover, they produce the volatile compound such as acetoin, which triggered the ISR. In A. thaliana, B. subtilis UMAF6639 restricts pathogen dissemination and disease progression in its aerial parts through ET- and SA-dependent and JA-independent response45. Cyclic lipopeptides such as iturins and fengycins from B. subtilis acting as potent antibiotics in the biocontrol of the tomato wilt disease caused by the phytopathogenic bacterium Ralstonia solanacearum46. A change in a JA receptor resulted in the sufficient signal transduction, fertility and defense conflicting insects, in addition to resistance in opposing P. syringae pathogenic strains47. Hence, the recent identification of host target modification could be a promising approach to protect plants from pathogen attack. Thus B. subtilis acts a significant antagonist in field conditions both in the external environment and endophytic colonization thereby contributing to ISR in eliciting a host defense response for increased productivity and devoid of loss due to phytopathogens,
VitB6 possess antioxidant activity and can modulate plant defense by regulating antioxidant status in plants48. However, collaborating VitB6 biosynthesis with biocontrol, disease resistance, ISR, stress alleviation, and tolerance is still absent with regard to B. subtilis CBR05 against XCV. In the present study, we examined B. subtilis CBR05 induced VitB6 biosynthetic genes in tomato confronted with XCV. Earlier up to the literature collected it was positively corroborated this is the first report involving B. subtilis CBR05 induced expression of VitB6 biosynthetic genes in tomato against XCV apart from biocontrol proficiency, ISR and plant improvement based on growth characters. We affirm that the present study could be rationalized for similar diseases not only in tomato but can be extended to other crops belonging to the Solanaceae family. The study would be a launch pad in further characterization and involvement of crosstalk between the gene regulation of de novo and salvage pathway in ISR mediated by B. subtilis.
Results
VitB6 biosynthetic genes expression and changes in VitB6 content
Expression profiling patterns of both de novo (PDX1 [PDX1.2 and 1.3] and PDX2), and salvage (SOS4) pathway genes in tomato plants after infection with XCV was undertaken as the primary rationale of the present study. PDX1.2, PDX1.3, PDX2.0, and SOS4 gene expression levels were compared at different time intervals in XCV and pyridoxine inoculated plants and was found to be significantly induced with different patterns in B. subtilis-inoculated plants (Fig. 1). After inoculation with B. subtilis, expression levels of PDX1.2 increased significantly at 48 and 72 hours post inoculation (hpi), in both XCV + B. subtilis and XCV + B. subtilis + pyridoxine, inoculated plants. In addition, B. subtilis and pyridoxine inoculation showed more than 5-fold increase than those in XCV inoculated plants at 72 hpi. Moreover, the transcript abundance of PDX1.3 in B. subtilis and pyridoxine inoculated plants significantly increased more than 12-fold higher than those in XCV inoculated plants at 72 hpi. PDX2 transcripts reached approximately 2.3-fold (XCV + B. subtilis) and 2.6-fold (XCV + B. subtilis + pyridoxine) increased over the pathogen control at 48 hpi, and then declined, reaching control expression level at 72 hpi. The results affirm that B. subtilis inoculation is responsible for the overexpression of VitB6 biosynthetic genes. PDX1.2 and PDX1.3 expression profiling show an escalated expression of pyridoxine at 72 hpi. On the contrary, PDX2 depict the overexpression in 72 hpi showing differential regulation. SOS4 gene expression profiling demarcates to nullified results without any significant outcomes. PDX1.2, PDX1.3, PDX2 genes overexpression confirms the authentic involvement of the de novo pathway of VitB6 biosynthesis in tomato upon XCV infection. On the other hand, SOS4 involving in salvage pathway was not significantly identified for expression patterns questioning how salvage pathway could possibly play a definitive regulatory role in oxidative stress tolerance.
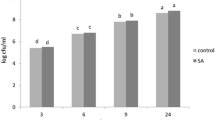
Relative fold-change expression of vitamin B6 biosynthetic genes in Xanthomonas campestris pv. vesicatoria-infected tomato plants. Quantitative real-time PCR analysis revealed de novo genes, including (a) PDX1.2, (b) PDX1.3 and (c) PDX2 and salvage pathway gene, (d) SOS4. Values were normalized to the values of the mock control. Data are means of triplicates ± SD and different letters are significant differences at P ≤ 0.05 level.
VitB6 contents in the plants without XCV inoculation and with XCV were examined to assess B. subtilis-induced expression of the VitB6 biosynthetic genes. VitB6 contents in pathogen-inoculated plants were lower than that in the mock-inoculated plants, showing reduction of 14, 6, and 15% for XCV, XCV + B. subtilis, and XCV + pyridoxine, respectively, at 24 hpi, as compared to those of mock-inoculated plants. These results, in consort with the fact that the transcript abundance of VitB6 gene in tomato after infection by XCV, show that reduced biosynthesis and hence reduced VitB6 content. It might be a natural defense response of plants to pathogenic infection as a part of defense mechanism. However, the VitB6 contents in the XCV + pyridoxine + B. subtilis inoculated plants, showing increase of 12, 93 and 76% at 24, 48 and 72 hpi, respectively, compared to that of mock-inoculated plants (Fig. 2). The result clearly shows that Bacillus inoculation has roles in VitB6 biosynthesis in all intervals indicating a way for profiling the specific genes. Differential regulation of the VitB6 genes could also involve a crosstalk between de novo and salvage pathway genes. Hence, further resistance mechanisms could positively associate with antioxidant profiling for establishing prominent disease resistance strategies.
XCV effect on superoxide dismutase (SOD) and catalase (CAT) activity
As shown in Fig. 3a, B. subtilis + XCV inoculated plants and XCV + pyridoxine inoculated plants had a significant increase in SOD activity at 24 hpi as compared to mock-inoculated plants (115 and 57%, respectively) and XCV inoculated plants (111 and 54%, respectively). We also found significant increase in SOD activity in the XCV + pyridoxine + B. subtilis inoculated plants, compared them with those in mock-inoculated pants (82%) and XCV inoculated plants (54%), at 24 hpi followed by decrease in SOD activity at 48–72 hpi (Supplementary Table S1). However, the activity of CAT in tomato plants increased rapidly after pathogen inoculation. CAT activity was significantly higher in the XCV + B. subtilis, XCV + pyridoxine and XCV + pyridoxine + B. subtilis by 44, 34 and 34%, respectively at 24 hpi, compared to those in XCV inocualted plants (Fig. 3b). We did not find any significant difference in the SOD and CAT activity at 48 hpi both in B. subtilis and X. campestris inoculated plants. Reduced levels of SOD and CAT activity was observed in XCV and pyridoxine inoculated plants at 48–72 hpi.
XCV effect on polyphenol oxidase (PPO) and peroxidase (POD) activity
Patterns of increase in PPO activity in the leaves during 24–72 hpi are illustrated in Fig. 3c. In, B. subtilis + XCV inoculated plants had a noteworthy increase in PPO activity at 48 hpi as compared to mock-inoculated controls (120%), XCV alone (4%), and XCV + pyridoxine (42%) inoculated plants. However, the PPO activity was significantly higher in the XCV + pyridoxine + B. subtilis inoculated plants, showing an increase of 18% compared with that in the XCV inoculated plants at 48 hpi. As shown in Fig. 3d, the treatment groups had a significant increase in POD production as compared to mock inoculated plants at 24 and 48 hpi. In, B. subtilis inoculated plants had a significant increase in POD activity at 24–72 hpi as compared to XCV, pyridoxine, and mock-inoculated controls. Induction of POD activity in the leaves was observed during 24 hpi and followed by a decline at 48–72 hpi. At 48 hpi, XCV inoculated plants showing an increase of 78% total POD activity than the mock-inoculated healthy control. Pot trials also corroborate that B. subtilis compared to pyridoxine supplementation alone show biocontrol activities on the leaf (Fig. 4). Figure 5 shows that root length of B. subtilis and pyridoxine inoculated plants were greatly increased by co-inoculation. Among inoculation, XCV + pyridoxine + B. subtilis (23.5 cm), followed by B. subtilis + XCV (16.5 cm), XCV + pyridoxine (16.0 cm) and mock-inoculated plants (14.0 cm) showed an increase in root length. Whereas, XC inoculated plants showed decreased in root length (9.0 cm).
Inoculation results of different tomato plants leaf. (a) Leaf of a mock-inoculated plant. (b) Leaf of 100 mM Pyridoxine inoculated plant. (c) Leaf of Bacillus subtilis CBR05 inoculated plant. (d) Leaf of Xanthomonas campestris pv. vesicatoria inoculated plant. (e) Leaf of Xanthomonas campestris pv. vesicatoria and 100 mM pyridoxine inoculated plant. (f) Leaf of Xanthomonas campestris pv. vesicatoria and Bacillus subtilis CBR05 inoculated plants. (g) Leaf of Xanthomonas campestris pv. vesicatoria, Bacillus subtilis CBR05 and 100 mM pyridoxine inoculated plants.
Inoculation results of different tomato plants root. (a) The root of a mock-inoculated plant. (b) Root of 100 mM Pyridoxine inoculated plant. (c) Root of Bacillus subtilis CBR05 inoculated plant. (d) Root of Xanthomonas campestris pv. vesicatoria inoculated plant. (e) Root of Xanthomonas campestris pv. vesicatoria and 100 mM pyridoxine inoculated plant. (f) Root of Xanthomonas campestris pv. vesicatoria and Bacillus subtilis CBR05 inoculated plants. (g) Root of Xanthomonas campestris pv. vesicatoria, Bacillus subtilis CBR05 and 100 mM pyridoxine inoculated plants.
Discussion
VitB6 is a collective term for a group of six interconvertible compounds1,7. It is most notable for its contribution to amino acid, carbohydrate and fatty acid metabolism where it serves as a cofactor for enzymes48. Despite these facts, the effect of VitB6 on plant development, the mechanisms controlling its biosynthesis and stress tolerance are still poorly understood. Reactive oxygen species (ROS) quenching and antioxidant properties by VitB6 have been an extensively studied phenomenon. VitB6 vitamers have been affirmed as significant singlet oxygen quenchers in vitro49. Nevertheless, well documented in A. thaliana, it needs to be assessed for its definitive role50. Further, PDX1.2 serves its role in sustaining VitB6 under critical conditions and stabilizes PDX1s under abiotic stress51. The literature available for most of the crop plants shows the manifestation of both de novo and salvage pathways in VitB6 genes for enhanced disease resistance upon phytopathogens infection. Recently, Zhang et al.52 proved that Botrytis cinerea-infected tomato plants show the involvement of the de novo vitB6 biosynthetic pathway and not salvage pathway by affirming SlPDX1.2 and SlPDX1.3 genes but not SlSOS4 through gene silencing studies based on disease severity. Also, Ralstonia solani RsolPDX1, RsolPDX2, and RsolPLR were the three principal factors involved in the VitB6 pathway in R. solani AG353. Most of the literature pertaining to VitB6 biosynthesis addresses de novo pathway mostly but salvage pathway at rare circumstances, interestingly lack of introns were denoted for plant-pathogen induced response for synthesis18. Hence, we could not clearly find a gap in abridging salvage pathway in oxidative stress-induced plant defense and antioxidant properties. The present study further adds novel results for the environmentally useful bacteria in eliciting a plethora of optimal outcomes. A non-native DXP-dependent VitB6 pathway in B. subtilis for the production of pyridoxine was shown to be present earlier54. Till date, the role of B. subtilis for the production of pyridoxine or involvement in VitB6 biosynthesis have not been classically addressed. It has been made possible through altered mechanisms like metabolic engineering strategies54. The present study provides molecular insights into the cumulative plant growth promotion, ISR and de novo pathway involvement of VitB6 biosynthesis.
Transcript profiling indicates overexpression of all four VitB6 biosynthetic genes was increased under stress; whereas, differential expression patterns were observed. PDX1.2, and PDX1.3, were found to be overexpressed significantly after 72 hpi with pyridoxine and B. subtilis in XCV inoculated plants showing that optimal synthesis of VitB6 could be possibly corroborated to the involvement of de novo pathway in tomato after XCV. Most significant upregulation was observed in the transcript profile of PDX1.3, which showed more than 12-fold increase in expression at 72 hpi. We also found significant up regulation of PDX2 in XCV + B. subtilis and XCV + pyridoxine + B. subtilis inoculated plants, which were 3.054- and 3.44-fold, respectively, at 48 hpi. From our results, we can clearly infer that SOS4 expression has not been significant. Increased expression of PDX1.3, PDX1.3, and PDX2 levels in our study proves that B. subtilis inoculated plants has roles in balancing the VitB6 biosynthesis. The previous study also showed that in A. thaliana, PDX2 balancing B6 vitamer levels55. This possibly explains that de novo pathway predominates over the salvage pathway having mechanisms as a bypass. The absence of a salvage pathway may be due to a demarcated pathway as that of the non-native pathway. In a similar study by Torky56, it clearly shows that VitB6 primes defense response and disease resistance in Capsicum annum upon Tobacco Mosaic Virus (TMV) infection. We also hypothesize that B. subtilis plays similar roles in the emancipation of cohesive response as that of TMV. In addition, VitB6 contents in XCV inoculated plants slightly decreased compared to those in the mock-inoculated plants. Moreover, a slight increase in the VitB6 content in mock-inoculated tomato leaves may be responsive to stress or wounding caused by mock infiltration. These results indicate that expression of VitB6 biosynthetic genes in tomato down-regulated after infection with XCV and hence reduced VitB6 content might be a natural response of plants to pathogenic infection as a part of defense mechanism18.
Previous studies showed thatVitB6 vitamers can act as antioxidant in plants and may act as an important modulator of redox status during pathogen defense response12,18,49. When compared with those in the control plants, increased disease level and increased accumulation of SOD after infection with XCV was observed. The increase of SOD activity accelerated enzymatic conversion of the superoxide anion to H2O2 and led to further accumulation of H2O2 in the XCV inoculated plants. Hence, increased accumulation of H2O2 will lead to production of CAT and POD. We also found that activity of PPO was induced in XCV inoculated plants compared to those in the mock-inoculated control plants. Moreover, antioxidant activity was higher in B. subtilis inoculated compared to those of other treatments. Previous studies, the showed that enhanced activities of antioxidant enzymes in plant tissues are positively associated with ISR and plant disease suppression30,37,41. Moreover, antioxidant activity in mock-inoculated healthy control was consistently present in all the test plants. The enzyme activity in control plants increased over time after inoculation showing native antioxidant enzyme presence.
Further, tomato plants infiltrated with XCV showed increased disease symptoms compared to those infiltrated with biocontrol agent, B. subtilis CBR05. However, co-inoculation with B. subtilis showed increased plant growth and decreased disease severity. At the same time supplementation of VitB6 vitamers like pyridoxine show comparatively low levels in the VitB6 synthesis and plant growth properties. Our results showed that the pyridoxine treated plants appeared in yellow color. Thus, the changed coloration may result from changed pigmentation12. Moreover, leaves infiltrated with pyridoxine and XCV increased the severity of chlorosis and necrosis associated with disease. Further inoculation of pyridoxine with B. subtilis colonizes root in plants and expresses root parameters. These results suggest that a possible role of pyridoxine in promoting root cell division and elongation. In our study, it clearly shows the above phenomena wherein, B. subtilis CBR05 extensively promote root growth in length and altered health in tomato roots through induced systemic resistance57. Further, active principles in B. subtilis have been attributed to surfactin in wheat as an elicitor58. With this view, active principles of B. subtilis could possibly act as a launch pad for the development of novel elicitors from our pilot study. Interestingly it has been observed that B. subtilis remarkably show similar patterns of 100 mM pyridoxine supplementation. Our proposition stating B. subtilis to be adaptable tool invoke several questions for the application in field conditions. Hence, pot culture experiments guarantee the application modalities in plants. The omnipresence of B. subtilis owing to several competencies in suppressing plant pathogens has been extensively studied39,40,41. B. subtilis has been well documented for usage against Fusarium wilt, Sclerotina sclerotiorum, Pythium, Phytophthora, Rhizoctonia, Septoria, and Verticillium in tomato59. This was further characterized at the molecular level showing a distinct lineage for the B. subtilis with copious prominent roles in plant enhancement mechanisms. XCV infection in Chinese cabbage has been studied for plant growth promotion and ISR which indicate Bacillus sp. of predominance60. Nevertheless, Xanthomonas abatement along with numerous molecular dissection mechanisms and VitB6 biosynthesis is lacking. Our study makes this versatile B. subtilis to be utilized as a broad spectrum agriculturally important microorganism. The present study is a holistic approach to address the B. subtilis eluding XCV with multiple molecular insights.
Current research focuses on assorted approach in interpreting real-time expression patterns of B. subtilis CBR05 induced VitB6 biosynthetic genes against XCV in tomato. The advancement in identifying B. subtilis CBR05 is the inherent objective of the present study. PDX1.2, PDX1.3, and PDX2 are the overexpressed VitB6 biosynthetic genes denoting involvement of de novo pathway in VitB6 biosynthesis induced by B. subtilis CBR05 against XCV. However, genes involved in salvage pathway have been not regulated as like de novo pathway genes. This is the first report that B. subtilis induces expression of VitB6 biosynthesis and can be a versatile bacterium in multifarious roles in tomato plant upgraded productivity. The present study can be rationalized for any crop and VitB6 biosynthesis.
Materials and Methods
Microorganism and culture conditions
In the present study, Xanthomonas campestris pv. vesicatoria (XCV) KACC11154 obtained from Korean Agricultural Culture Collection (KACC), South Korea, was used as the phytopathogen. B. subtilis CBR05 used in this study were obtained from Prof. Se Chul Chun lab, used as a biocontrol agent. Bacterial cells were subcultured in tryptic soy broth. They were transferred to tryptic soy agar and incubated at 30 °C for 24 h. The bacterial cells were harvested and resuspended in 10 mM MgCl2. B. subtilis CBR05 viable population was adjusted to 108 CFU/ml for further studies.
Plant materials and growth conditions
We obtained seeds of tomato from Korea seed resource center. They were surface sterilized with 70% ethanol for 5 min, finally rinsed three times with sterile distilled water. Tomato seedlings were transferred into plastic pots after four weeks. The experiment was carried out in a randomized complete block design with three plants as replicates in each of the following treatments: (i) Mock (4% maltose and 1% peptone solution) (ii) XCV alone (iii) XCV + B. subtilis (iv) XCV + 100 mM pyridoxine (v) XCV + B. subtilis + 100 mM pyridoxine. Pots were incubated in a growth room. Three pots were maintained per treatment, each with a single plant and arranged in a completely randomized design with three replications.
Total RNA isolation and cDNA synthesis
Leaf samples were collected from tomato plants. Total RNA was isolated from frozen tomato tissue using the RNeasy Plant Mini Kit (Qiagen, Germany), according to manufacturer’s instructions. Genomic DNA contamination from RNA samples was removed by DNAse treatment. Formaldehyde agarose gel electrophoresis was used for the verification of the integrity of total RNA. We determined the purity and concentration of the total RNA by spectrophotometry (NanoDrop ND-1000 Spectrophotometer; Celbio, Italy). RNA samples were reverse transcribed into cDNA using First-strand cDNA synthesis kit (Bioneer, Korea).
Quantitative PCR (qPCR)
The synthesized cDNA was used as a template for real-time PCR reactions using the instrument CFX96™ Real-Time System (Bio-Rad, Hercules, CA, USA). The sequences of primers used in this study for RT-qPCR analysis were listed in Supplementary Table S2. Each reaction (25 μL) contained 12.5 μL of AccuPower® 2x Greenstar qPCR Master Mix (Bioneer, Korea), forward and reverse primer (0.4 μM), 5 μL of diluted cDNA sample and nuclease-free water. The qPCR reactions were carried out using the following PCR cycling conditions: 95 °C for 10 min (1 cycle), the 20 s at 95 °C followed by annealing and extension at 60 °C for 45 s (40 cycles). The actin gene was used as the internal reference for normalization. The expression level of the VitB6 gene transcripts was calculated using CFX Manager Software (Bio-Rad). All samples were analyzed with three independent total RNA samples.
Measurement of VitB6 content
VitB6 contents were determined using a bioassay with Yeast strain (Saccharomyces cerevisiae ATCC9080), auxotrophic for VitB6. Leaf samples were collected from each experiment and leaf extracts were prepared with a protocol as described earlier18. Cells were grown in Pyridoxine Y medium for 12 h at 30 °C, 220 rpm. Overnight culture was washed two times with Pyridoxine Y medium for removal of residual pyridoxine. One milliliter of leaf extract was added to tubes containing 5 × 108 yeast cells in 5 ml of Pyridoxine Y medium. Assay tubes were incubated for 16 h, at 30 C, 220 rpm. Yeast growth was measured by spectrophotometry at 540 nm. Pyridoxine was used as a standard.
Determination of antioxidant enzymes
Determination of SOD activity
SOD was determined using the SOD activity kit (Enzo Life Sciences Inc., USA), according to manufacturer’s instructions. The enzyme extract (25 μl) was added to 150 μl of Master Mix. The reaction was started by adding 25 μl of 1× xanthine solution to all the wells. The well without enzyme solution (added 25 μl of the 1× SOD buffer) were considered as a control. The total SOD activity was measured using a microtiter plate reader, optical density (OD) at 450 nm. The specific activity was expressed as units per milligram of protein (Units mg−1 protein).
Determination of CAT activity
The catalase fluorometric detection kit (Enzo-Catalog # ADI-907-027) used to detect CAT activity by measuring the amount of H2O2, according to the manufacturer’s protocol. The reaction mixture contained 50 μL of enzyme extract and 50 μL of 40 μM H2O2. They were incubated at room temperature for 60 min. After incubation, 100 μL of reaction cocktail was added to the mixture and incubated plate for 15 min. The consumption of H2O2 was monitored using excitation at 570 nm and measured fluorescence. The specific activity was expressed as Units mg−1 protein.
Determination of PPO activity
PPO activity was determined spectrophotometrically. The reaction mixture consisted of 200 μL enzyme extract and 1.5 mL of 0.1 M sodium phosphate buffer (pH 6.5). We added 200 μL of 0.01 M catechol to the reaction mixture. The enzyme activity was measured at 420 nm. The specific activity was expressed as Units mg−1 protein.
Determination of POD activity
POD activity was quantified by mixing the enzyme extract (50 μL) with 2.85 mL of 100 mM phosphate buffer (pH 7.0) and 20 mM guaiacol (50 μL). Followed by addition of 40 mM H2O2 (20 μL) to the reaction mixture. The oxidation reaction was measured by spectrophotometry at 470 nm. The specific activity was expressed as Units mg−1 protein.
Statistical analysis
All the experiments were conducted in triplicate and results were tabulated as the mean ± standard deviation (SD). Data were analyzed by analysis of variance (ANOVA). Student’s t-test, Duncan’s Multiple Range Test, and the probability values of P ≤ 0.05 were considered to be significant.
References
Fitzpatrick, T. B. et al. Two independent routes of de novo vitamin B6 biosynthesis: not that different after all. Biochem. J. 407, 1–13 (2007).
Dong, Y. X., Sueda, S., Nikawa, J. I. & Kondo, H. Characterization of the products of the genes SNO1 and SNZ1 involved in pyridoxine synthesis in Saccharomyces cerevisiae. Eur. J. Biochem. 271, 745–752 (2004).
Wetzel, D. K., Ehrenshaft, M., Denslow, S. A. & Daub, M. E. Functional complementation between the PDX1 vitamin B6 biosynthetic gene of Cercospora nicotianae and pdxJ of Escherichia coli. FEBS (Fed. Eur. Biochem. Soc.) Lett. 564, 143–146 (2004).
Tambasco-Studart, M., Tews, I., Amrhein, N. & Fitzpatrick, T. B. Functional analysis of PDX2 from Arabidopsis, a glutaminase involved in vitamin B6 biosynthesis. Plant Physiol. 144, 915–925 (2007).
Benabdellah, K. et al. GintPDX1 encodes a protein involved in vitamin B6 biosynthesis that is up‐regulated by oxidative stress in the arbuscular mycorrhizal fungus Glomus intraradices. New Phytologist 184, 682–693 (2009).
Drewke, C. & Leistner, E. Biosynthesis of vitamin B6 and structurally related derivatives. Vitam. Horm. 61, 121–155 (2001).
Mooney, S., Leuendorf, J. E., Hendrickson, C. & Hellmann, H. Vitamin B6: A long known compound of surprising complexity. Molecules 14, 329–351 (2009).
Shi, H. & Zhu, J. K. SOS4, a pyridoxal kinase gene, is required for root hair development in Arabidopsis. Plant Physiol. 129, 585–593 (2002).
González, E., Danehower, D. & Daub, M. E. Vitamer levels, stress response, enzyme activity, and gene regulation of Arabidopsis lines mutant in the pyridoxine/pyridoxamine 5′-phosphate oxidase (PDX3) and the pyridoxal kinase (SOS4) genes involved in the vitamin B6 salvage pathway. Plant Physiol. 145, 985–996 (2007).
Herrero, S., González, E., Gillikin, J. W., Vélëz, H. & Daub, M. E. Identification and characterization of a pyridoxal reductase involved in the vitamin B6 salvage pathway in Arabidopsis. Plant Mol. Biol. 76, 157–169 (2011).
Rueschhoff, E. E., Gillikin, J. W., Sederoff, H. W. & Daub, M. E. The SOS4 pyridoxal kinase is required for maintenance of vitamin B6- mediated processes in chloroplasts. Plant Physiol. Biochem. 63, 281–291 (2013).
Chen, H. & Xiong, L. Pyridoxine is required for post-embryonic root development and tolerance to osmotic and oxidative stress. Plant J. 44, 396–408 (2005).
Titiz, O. et al. PDX1 is essential for vitamin B6 biosynthesis, development and stress tolerance in Arabidopsis. Plant J. 48, 933–946 (2006).
Denslow, S. A., Rueschhoff, E. E. & Daub, M. E. Regulation of the Arabidopsis thaliana vitamin B6 biosynthesis genes by abiotic stress. Plant Physiol. 45, 152–161 (2007).
Havaux, M. et al. Vitamin B6 deficient plants display increased sensitivity to high light and photo-oxidative stress. BMC Plant Biol. 9, 130 (2009).
Leuendorf, J. E., Osorio, S., Szewczyk, A., Fernie, A. R. & Hellmann, H. Complex assembly and metabolic profiling of Arabidopsis thaliana plants overexpressing vitamin B6 biosynthesis proteins. Mol. Plant 3, 890–903 (2010).
Raschke, M. et al. Enhanced levels of vitamin B6 increase aerial organ size and positively affect stress tolerance in Arabidopsis. Plant J. 66, 414–432 (2011).
Denslow, S. A., Walls, A. A. & Daub, M. E. Regulation of biosynthetic genes and antioxidant properties of vitamin B6 vitamers during plant defense responses. Physiol. Mol. Plant Pathol. 66, 244–255 (2005).
Sivasubramaniam, S., Vanniashingham, V. M., Tan, C. T. & Chua, N. H. Characterization of HEVER, a novel stress-induced gene from Hevea brasiliensis. Plant Mol. Biol. 29, 173–178 (1995).
Jones, J. B., Lacy, G. H., Bouzar, H., Stall, R. E. & Schaad, N. W. Reclassification of the Xanthomonads associated with bacterial spot disease of tomato and pepper. Syst. Appl. Microbiol. 27, 755–762 (2004).
Potnis, N. et al. Bacterial spot of tomato and pepper: diverse Xanthomonas species with a wide variety of virulence factors posing a worldwide challenge. Mol. Plant Pathol. 16, 907–920 (2015).
Schwartz, A. R. et al. Phylogenomics of Xanthomonas field strains infecting pepper and tomato reveals diversity in effector repertoires and identifies determinants of host specificity. Front. Microbiol. 6, 535–00 (2015).
Timilsina, S. et al. Multilocus sequence analysis of Xanthomonads causing a bacterial spot of tomato and pepper plants reveals strains generated by recombination among species and recent global spread of Xanthomonas gardneri. Appl. Environ. Microbiol. 81, 1520–1529 (2015).
Wu, L. et al. Difficidin and bacilysin from Bacillus amyloliquefaciens FZB42 have antibacterial activity against Xanthomonas oryzae rice pathogens. Sci. Rep. 5, 12975 (2015).
Denancé, N. et al. Two ancestral genes shaped the Xanthomonas campestris TAL effector gene repertoire. New Phytologist 219, 391–407 (2018).
Bardin, M. et al. Is the efficacy of biological control against plant diseases likely to be more durable than that of chemical pesticides? Front. Plant Sci. 6, 566 (2015).
Saha, D., Purkayastha, G. D., Ghosh, A., Isha, M. & Saha, A. Isolation and characterization of two new Bacillus subtilis strains from the rhizosphere of eggplant as potential biocontrol agents. J. Plant Pathol. 94, 109–118 (2012).
Kloepper, J. W. et al. Plant root–bacterial interactions in biological control of soil borne diseases and potential extension to systemic and foliar diseases. Australas. Plant Pathol. 28, 21–26 (1999).
Moss, W. P. et al. Biological control of bacterial spot of tomato using hrp mutants of Xanthomonas campestris pv. vesicatoria. Biol. Control 41, 199–206 (2007).
Ferraz, H. G. M. et al. Rhizobacteria induces resistance against Fusarium wilt of tomato by increasing the activity of defense enzymes. Bragantia 73, 274–283 (2014).
Yim, W. J. et al. Real time expression of ACC oxidase and PR-protein genes mediated by Methylobacterium spp. in tomato plants challenged with Xanthomonas campestris pv. vesicatoria. J. Plant Physiol. 171, 1064–1075 (2014).
Areas, M. S. et al. Prevalence of Xanthomonas euvesicatoria on pepper in Brazil. J Phytopathol. 163, 1050–1054 (2015).
Kloepper, J. W., Ryu, C. M. & Zhang, S. A. Induced systemic resistance and promotion of growth by Bacillus spp. Phytopathol. 94, 1259–1266 (2004).
Byrne, J. M. et al. Biological control of bacterial spot of tomato under field conditions at several locations in North America. Biol. Control 32, 408–418 (2005).
Van Loon, L. C. Plant responses to plant growth-promoting rhizobacteria. Eur. J. Plant Pathol. 119, 243–254 (2007).
Ferraz, H. G. M. et al. Antagonistic rhizobacteria and jasmonic acid induce resistance against tomato bacterial spot. Bragantia 74, 417–427 (2015).
Chandrasekaran, M., Belachew, S. T., Yoon, E. & Chun., S. C. Expression of β-1,3-glucanase (GLU) and phenylalanine ammonia-lyase (PAL) genes and their enzymes in tomato plants induced after treatment with Bacillus subtilis CBR05 against Xanthomonas campestris pv. vesicatoria. J. Gen. Plant Pathol. 83, 7–13 (2017).
Pieterse, C. M. J. et al. Induced systemic resistance by beneficial microbes. Annu. Rev. Phytopathol. 52, 347–375 (2014).
Chithrashree, R., Udayasankar, A. C. & Nayaka, S. C. Plant growth promoting rhizobacteria mediate induced systemic resistance in rice against bacterial leaf blight caused by Xanthomonas oryzae pv. oryzae. Biol. Control 59, 114–122 (2011).
Lanna, R. F. et al. Biocontrol activity of Bacillus against a GFP-marked Pseudomonas syringae pv. tomato on tomato phylloplane. Australas. Plant Pathol 42, 643–651 (2013).
Chandrasekaran, M. & Chun, S. C. Expression of PR-protein genes and induction of defense-related enzymes by Bacillus subtilis CBR05 in tomato (Solanum lycopersicum) plants challenged with Erwinia carotovora subsp. carotovora. Biosci. Biotechnol. Biochem. 80, 2277–2283 (2016).
Ryan, R. P., Germaine, K., Franks, A., Ryan, D. J. & Dowling, D. N. Bacterial endophytes: Recent developments and applications. FEMS Microbiol. Lett. 278, 1–9 (2008).
Zamioudis, C. & Pieterse, C. M. J. Modulation of host immunity by beneficial microbes. Molecular Plant-Microbe Interactions 25, 139–150 (2012).
Rudrappa, T. et al. The rhizobacterial elicitor acetoin induces systemic resistance in Arabidopsis thaliana. Commun. Integr. Biol. 3, 130–138 (2010).
García-Gutiérrez, L. et al. The antagonistic strain Bacillus subtilis UMAF6639 also confers protection to melon plants against cucurbit powdery mildew by activation of jasmonate-and salicylic acid-dependent defence responses. Microb. Biotechnol. 6, 264–274 (2013).
Chen, Y. et al. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ. Microbiol. 15, 848–864 (2013).
Zhang, L. et al. Host target modification as a strategy to counter pathogen hijacking of the jasmonate hormone receptor. Proc. Natl. Acad. Sci. USA 112, 14354–14359 (2015).
Vanderschuren, H. et al. Strategies for vitamin B6 biofortification of plants: A dual role as a micronutrient and a stress protectant. Front. Plant Sci. 4, 143 (2013).
Bilski, P., Li, M. Y., Ehrenshaft, M., Daub, M. E. & Chignell, C. F. Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem. Photobiol. 71, 129–134 (2000).
Moccand, C. et al. The pseudoenzyme PDX1.2 boosts vitamin b6 biosynthesis under heat and oxidative stress in. Arabidopsis. J. Biol. Chem. 289, 8203–8216 (2014).
Dell’Aglio, E., Boycheva, S. & Fitzpatrick, T. B. The pseudoenzyme pdx1.2 sustains vitamin b6 biosynthesis as a function of heat stress. Plant Physiol. 174, 2098–2112 (2017).
Zhang, Y. et al. The de novo biosynthesis of vitamin B6 is required for disease resistance against Botrytis cinerea in tomato. Mol. Plant Microbe Interact. 27, 688–699 (2014).
Samsatly, J., Chamoun, R., Gluck-Thaler, E. & Jabaji, S. Genes of the de novo and salvage biosynthesis pathways of vitamin B6 are regulated under oxidative stress in the plant pathogen Rhizoctonia solani. Front. Microbiol. 6, 1429 (2016).
Commichau, F. M. et al. Overexpression of a non-native deoxyxylulose-dependent vitamin B6 pathway in Bacillus subtilis for the production of pyridoxine. Metab Eng 25, 38–49 (2014).
Colinas, M. et al. Balancing of B6 vitamers is essential for plant development and metabolism in Arabidopsis. The Plant Cell 28, 439–453 (2016).
Torky, A. Z. Vitamin B Mediated Priming of Disease Resistance and Defense Responses to Tobacco Mosaic Virus in Capsicum annuum L. Plants. J Antivir Antiretrovir 8, 035–053 (2016).
Choudhary, D. K. & Johri, B. N. Interactions of Bacillus spp. and plants–with special reference to induced systemic resistance (ISR). Microbiol. Res. 164, 493–513 (2009).
Ongena, M. et al. Surfactin and fengycin lipopeptides of Bacillus subtilis as elicitors of induced systemic resistance in plants. Environ. Microbiol. 9, 1084–1090 (2007).
Shafi, J., Tian, H. & Ji, M. Bacillus species as versatile weapons for plant pathogens: a review. Biotechnol. Biotechnol. Equip. 31, 446–459 (2017).
Liu, K., Garrett, C., Fadamiro, H. & Kloepper, J. W. Induction of systemic resistance in Chinese cabbage against black rot by plant growth promoting rhizobacteria. Biol. Control 99, 8–13 (2016).
Author information
Authors and Affiliations
Contributions
M.C. and S.C. conceived and designed study; M.C. performed the experiments; M.C. and M.P wrote the manuscript with assistance from S.C. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Chandrasekaran, M., Paramasivan, M. & Chun, SC. Bacillus subtilis CBR05 induces Vitamin B6 biosynthesis in tomato through the de novo pathway in contributing disease resistance against Xanthomonas campestris pv. vesicatoria. Sci Rep 9, 6495 (2019). https://doi.org/10.1038/s41598-019-41888-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41888-6
- Springer Nature Limited
This article is cited by
-
Mechanisms of probiotic Bacillus against enteric bacterial infections
One Health Advances (2023)
-
Paenibacillus polymyxa NSY50 Improves Defense Against Fusarium oxysporum by Increasing Photosynthetic Efficiency, Sucrose Metabolism, and Antioxidant Capacity in Cucumber
Journal of Plant Growth Regulation (2023)
-
Biocontrol of bacterial spot on tomato by foliar spray and growth medium application of Bacillus amyloliquefaciens and Trichoderma asperellum
European Journal of Plant Pathology (2020)