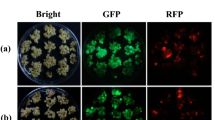
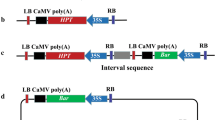
Abstract
The use of particle gun for the production of marker-free plants is scant in published literature. Perhaps this is a reflection of the widely held notion that the events generated through bombardment tend to have multiple copies of transgenes, usually integrated at a single locus, features which precludes segregating away the selectable marker gene. However, our previous studies have shown that single-copy integrants are obtained at a high frequency if limited quantity of DNA is used for bombardment. Also, the concatemerized insertion of transgenes has been demonstrated to be greatly reduced if “cassette DNA” is employed in place of whole plasmid DNA for bombardment. Based on the above findings, in the present study the feasibility of co-bombardment was evaluated for the production of marker-free plants in corn, employing a combination of limited quantity DNA and cassette DNA approaches for bombardment. Transgenic events were generated after co-bombardment of a selectable marker cassette containing the nptII gene (2.5 ng per shot) and a GUS gene cassette (15 ng per shot). Among these events single-copy integrants for nptII gene occurred at an average frequency of 68% within which the co-expression frequency of GUS and nptII genes ranged from 41% to 80%. Marker-free corn plants could be identified from the progeny of 28 out of the 103 R0 co-expressing events screened. The results demonstrate that by using cassette DNA and low quantities of DNA for bombardment, marker-free plants are produced at efficiencies comparable to that of Agrobacterium-based co-transformation methods.



Similar content being viewed by others
Abbreviations
- P-CaMV.35S:
-
Cauliflower mosaic virus 35S promoter
- GUS :
-
β-glucuronidase
- nptII :
-
Neomycin phosphotransferase II
- nos:
-
Nopaline synthase
- Bt :
-
Bacillus thuringiensis
- P-Os.rbcS:
-
Oryza sativa rubisco small subunit promoter
- P-Os.Actin15:
-
Oryza sativa actin15 promoter
- P-Os.GT-1:
-
Oryza sativa glutelin promoter
- GOI:
-
Gene of interest
- LWA:
-
Leaf whorl assay
- DAG:
-
Days after germination
References
Agrawal PK, Ajay K, Twyman RM, Christou P (2005) Transformation of plants with multiple cassettes generates simple transgene integration patterns and high expression levels. Mol Breed 16:247–260
Altpeter F, Vasil V, Srivastava V, Vasil IK (1996) Integration and expression of the high-molecular-weight glutenin subunit 1Ax1 gene into wheat. Nat Biotechnol 14:1155–1159
Altpeter F, Baisakh N, Beachy R, Bock R, Capell T, Christou P, Daniell H, Datta K, Datta S, Dix PJ, Fauquet C, Huang N, Kohli A, Mooibroek H, Nicholson L, Nguyen TH, Nugent G, Raemakers K, Romano A, Somers DA, Stoger E, Taylor N, Visser R (2005) Particle bombardment and the genetic enhancement of crops: myths and realities. Mol Breed 15:305–327
Aragao FJL, Brasileiro ACM (2002) Positive, negative and marker-free strategies for transgenic plant selection. Br J Plant Physiol 14:1–10
Aragao FJL, Barros LMG, Brasileiro ACM, Ribeiro SG, Smith FD, Sanford JC, Faria JC, Rech EL (1996) Inheritance of foreign genes in transgenic bean (Phaseolus vulgaris L.) co-transformed via particle bombardment. Theor Appl Genet 93:142–150
Chen L, Marmey P, Taylor NJ, Brizard JP, Espinoza C, D’Cruz P, Huet H, Zhang S, Kochko A, Beachy RN, Fauquet CM (1998) Expression and inheritance of multiple transgenes in rice plants. Nat Biotechnol 16:1060–1064
Dai S, Zheng P, Marmey P, Zhang S, Tian W, Chen S, Beachy RN, Fauquet C (2001) Comparative analysis of transgenic rice plants obtained by Agrobacterium-mediated transformation and particle bombardment. Mol Breed 7:25–33
Daley M, Knauf VC, Summerfelt KR, Turner JC (1998) Co-transformation with one Agrobacterium tumefaciens strain containing two binary plasmids as a method for producing marker-free transgenic plants. Plant Cell Rep 17:489–496
Darbani B, Eimanifar A, Stewart CN, Camargo WN (2007) Methods to produce marker-free transgenic plants. Biotechnol J 2:83–90
De Block M, Debrouwer D (1991) Two T-DNA’s co-transformed in to Brassica napus by a double Agrobacterium tumefaciens infection are mainly integrated at the same locus. Theor Appl Genet 82:257–263
Depicker A, Herman L, Schell J, Van Montagu M (1985) Frequencies of simultaneous transformation with different T-DNAs and their relevance to the Agrobacterium plant cell interaction. Mol Gen Genet 201:477–484
Dong J, Kharb P, Teng W, Hall TC (2001) Characterization of rice transformed via an Agrobacterium-mediated inflorescence approach. Mol Breed 7:187–194
Fu X, Duc LT, Fontana S, Bong BB, Tinjuangjun P, Sudhakar D, Twyman RM, Christou P, Kohli A (2000) Linear transgene constructs lacking vector backbone sequences generate low-copy-number transgenic plants with simple integration patterns. Transgenic Res 9:11–19
Gadaleta A, Giancaspro A, Blechl AE, Blanco A (2008) Transgenic durum wheat line that is free of marker genes and expresses 1Dy10. J Cereal Sci 48:439–445
Gupta M, Nirunsuksiri W, Schulenberg G, Hartl T, Novak S, Bryan J, Vanopdorp N, Bing J, Thompson S (2008) A non-PCR-based Invader® assay quantitatively detects single-copy genes in complex plant genomes. Mol Breed 21:173–181
Hiei Y, Ohta S, Komari T, Kumashiro T (1994) Efficient transformation of rice (Oryza sativa L.) mediated by Agrobacterium and sequence analysis of the boundaries of the T-DNA. Plant J 6:271–282
Howe A, Sato S, Dweikat I, Fromm M, Clemente T (2006) Rapid and reproducible Agrobacterium-mediated transformation of sorghum. Plant Cell Rep 25:784–791
Huang S, Gilbertson LA, Adams TH, Malloy KP, Reisenbigler EK, Birr DH, Snyder MW, Zhang Q, Luethy MH (2004) Generation of marker-free transgenic maize by regular two-border Agrobacterium transformation vectors. Transgenic Res 13:451–461
Ishida Y, Saito H, Ohta S, Hiei Y, Komari T, Kumashiro T (1996) High efficiency transformation of maize (Zea mays L.) mediated by Agrobacterium tumefaciens. Nat Biotechnol 14:745–750
Jacob SS, Veluthambi K (2002) Generation of selection marker-free transgenic plants by co-transformation of a cointegrate vector T-DNA and a binary vector T-DNA in one Agrobacterium tumefaciens strain. Plant Sci 163:801–806
Jefferson RA (1987) Assaying chimeric genes in plants: the GUS gene fusion system. Plant Mol Biol Report 5:387–405
Kim SR, Lee J, Jun SH, Park S, Kang HG, Kwon S, An G (2003) Transgene structures in T-DNA-inserted rice plants. Plant Mol Biol 52:761–773
Kohli A, Leech M, Vain P, Laurie DA, Christou P (1998) Transgene organization in rice engineered through direct DNA transfer supports a two-phase integration mechanism mediated by the establishment of integration hot spots. Proc Natl Acad Sci USA 95:7203–7208
Komari T, Hiei Y, Saito Y, Murai N, Kumashiro T (1996) Vectors carrying two separate T-DNAs for cotransformation of higher plants mediated by Agrobacterium tumefaciens and segregation of transformants free from selection markers. Plant J 10:165–174
Lowe BA, Shiva Prakash N, Way M, Mann MT, Spencer TM, Boddupalli RS (2009) Enhanced single copy integration events in corn via particle bombardment using low quantities of DNA. Transgenic Res. doi:10.1007/s11248-009-9265-0
McCabe D, Christou P (1993) Direct DNA transfer using electrical discharge particle acceleration (ACCELL™ technology). Plant Cell Tissue Org Cult 33:227–236
McCormac AC, Fowler MR, Chen DF, Elliott MC (2001) Efficient co-transformation of Nicotiana tabacum by two independent T-DNAs, the effect of T-DNA size and implications for genetic separation. Transgenic Res 10:143–155
Miki B, McHugh S (2004) Selectable marker genes in transgenic plants: applications, alternatives and biosafety. J Biotechnol 107:193–232
Miller M, Tagliani L, Wang N, Berka B, Bidney D, Zhao ZY (2002) High efficiency transgene segregation in co-transformed maize plants using an Agrobacterium tumefaciens 2 T-DNA binary system. Transgenic Res 11:381–396
Puchta H (2003) Marker-free transgenic plants. Plant Cell Tissue Org Cult 74:123–134
Romano A, Raemakers K, Bernardi J, Visser R, Mooibroek H (2003) Transgene organization in potato after particle bombardment-mediated (co)transformation using plasmids and gene cassettes. Transgenic Res 12:461–473
Rooke L, Steele SH, Barcelo P, Shewry PR, Lazzeri PA (2003) Transgene inheritance, segregation and expression in bread wheat. Euphytica 129:301–309
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Shiva Prakash N, Prasad V, Chidambram TP, Cherian S, Jayaprakash TL, Dasgupta S, Wang Q, Mann MT, Spencer TM, Boddupalli RS (2008) Effect of promoter driving selectable marker on corn transformation. Transgenic Res 17:695–704
Shou H, Frame BR, Whitham SA, Wang K (2004) Assessment of transgenic maize events produced by particle bombardment or Agrobacterium-mediated transformation. Mol Breed 13:201–208
Tang K, Tinjuangjun P, Xu Y, Sun X, Gatehouse JA, Ronald PC, Qi H, Lu X, Christou P, Kohli A (1999) Particle bombardment-mediated co-transformation of elite Chinese rice cultivars with genes conferring resistance to bacterial blight and sap-sucking insect pests. Planta 208:552–563
Tingay S, McElroy D, Kalla R, Fieg S, Wang M, Thornton S, Brettell R (1997) Agrobacterium tumefaciens-mediated barley transformation. Plant J 11:1369–1376
Tobias DJ, Manoharan M, Pritsch C, Dahleen LS (2007) Co-bombardment, integration and expression of rice chitinase and thaumatin-like protein genes in barley (Hordeum vulgare cv. Conlon). Plant Cell Rep 26:631–639
Tu J, Datta K, Oliva N, Zhang G, Xu C, Khush GS, Zhang Q, Datta SK (2003) Site-independently integrated transgenes in the elite restorer rice line Minghui 63 allow removal of a selectable marker from the gene of interest by self-segregation. Plant Biotech J 1:155–165
Vain P, Afolabi AS, Worland B, Snape JB (2003) Transgene behaviour in populations of rice plants transformed using a new dual binary vector system: pGreen/pSoup. Theor Appl Genet 107:210–217
Verweire D, Verleyen K, De-Buck S, Claeys M, Angenon G (2007) Marker-free transgenic plants through genetically programmed auto-excision. Plant Physiol 145:1220–1231
Wang D, Zhao Q, Zhu D, Ao G, Yu J (2006) Particle-bombardment-mediated co-transformation of maize with a lysine rich protein gene (sb401) from potato. Euphytica 150:75–85
Wu L, Nandi S, Chen L, Rodriguez RL, Huang N (2002) Expression and inheritance of nine transgenes in rice. Transgenic Res 11:533–541
Yin Z, Wang GL (2000) Evidence of multiple complex patterns of T-DNA integration into the rice genome. Theor Appl Genet 100:461–470
Yoder JI, Goldsbrough AP (1994) Transformation systems for generating marker-free transgenic plants. Bio/Technol 12:263–267
Zhao ZY, Gu W, Cai T, Tagliani LA, Hondred DA, Bond D, Krell S, Rudert ML, Bruce WB, Pierce DA (1998) Molecular analysis of T0 plants transformed by Agrobacterium and comparison of Agrobacterium-mediated transformation with bombardment transformation in maize. Maize Genet Coop Newsl 72:34–37
Zhao Y, Qian Q, Wang H-Z, Huang D-N (2007) Co-transformation of gene expression cassettes via particle bombardment to generate safe transgenic plant without any unwanted DNA. In Vitro Cell Dev Biol Plant 43:328–334
Acknowledgments
Many people have contributed to these investigations carried out by the corn transformation and greenhouse teams at the Monsanto Research Centre, Bangalore, India. We are grateful to James D. Massucci, James F. Rice and Rodney A. Kowalewski, Monsanto Company, St. Louis, MO, USA, for performing radio-active Southern hybridization blots. We thank Beiyan Zeng, Monsanto Company, St. Louis, MO, USA, for her help with statistical analysis. The technical assistance rendered by Venkatachalapathy Muniraju and Janardhana Sundupalle is acknowledged. The authors are indebted to Mittur N. Jagadish for his encouragement throughout this study and for the critical reading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by P. Kumar.
Rights and permissions
About this article
Cite this article
Shiva Prakash, N., Bhojaraja, R., Shivbachan, S.K. et al. Marker-free transgenic corn plant production through co-bombardment. Plant Cell Rep 28, 1655–1668 (2009). https://doi.org/10.1007/s00299-009-0765-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00299-009-0765-4