Abstract
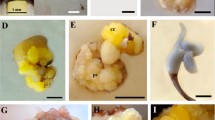
Olive is one of the most important oil crops in the Mediterranean area. Biotechnological improvement of this species is hampered by the recalcitrant nature of olive tissue regeneration in vitro. In this investigation, we have developed an efficient regeneration system for juvenile olive explants via somatic embryogenesis. Embryogenic cultures were obtained at a rate of 25% by culturing isolated radicles from mature seeds in a modified olive medium (OMc) containing 2.5 μM 6-(dimethylallylamino) purine (2iP) and 25 μM indole-3-butyric acid (IBA) over 3 weeks and later transferring to the same medium without 2iP and with a lower IBA concentration. Two different basal formulations, OMc and olive cyclic embryogenesis medium (ECO) [1/4 OM macroelements, 1/4 Murashige and Skoog (MS) microelements and 1/2 OM vitamins supplemented with 550 mg l−1 glutamine], were tested for embryogenic callus proliferation and maturation. The growth rate of embryogenic calli was similar in both media. However, the regeneration of mature embryos, achieved by culturing embryogenic masses in the same medium without hormones and supplemented with 1 g l−1 activated charcoal, was significantly higher when embryos were cultured in the ECO basal formulation. Pre-culturing embryogenic masses in liquid medium for up to 4 weeks did not affect subsequent callus proliferation in solid medium. The maturation rate of small globular somatic embryos, 1–3 mm size, obtained after filtering liquid cultures through a 3 × 3 mm mesh, was also similar to control embryos cultured in solid medium. To improve the maturation and germination rates, the effect of culturing globular somatic embryos on semi-permeable cellulose acetate membranes was also tested. Membrane treatments reduced the regeneration of mature embryos from 56.5% in the control treatment to 40.6% when the membrane was applied during the first half of the 8-week maturation phase and to 18% when the membrane was applied during last 4 weeks of the maturation period. However, membrane treatments significantly enhanced the conversion of mature embryos to plants, increasing the embryo conversion rate from 1.5% in the control to an average value of 37.8% in the membrane treatment. Cotyledonary embryos that were matured on the membranes showed lower values of water and solute potential than controls, indicating that this treatment exerted a controlled desiccation rate that enhanced the recovery of plants.



Similar content being viewed by others
Abbreviations
- 2iP:
-
6-(Dimethylallylamino) purine
- BA:
-
6-Benzyladenine
- DKW:
-
Driver and Kuniyuki medium
- ECO:
-
Olive cyclic embryogenesis medium
- IBA:
-
Indole-3-butyric acid
- MS:
-
Murashige and Skoog
- OMc:
-
Modified olive medium
- SE:
-
Somatic embryo
References
Bhradda N, Abousalim A, Walali LDM (2003) Effects du milieu de culture et de la lumière sur l’embryogenèse somatique de l’olivier (Olea europaea L.) cv. Picholine marocaine. Fruits 58:167–174
Bourgin JP, Nitsch JP (1967) Obtention de Nicotiana haploïdes a partir d’étamines cultivées in vitro. Ann Physiol Veg 9:377–382
Braybrook SA, Harada JJ (2008) LECs go crazy in embryo development. Trends Plant Sci 13:624–630
Cañas LA, Benbadis A (1988) In vitro plant regeneration from cotyledon fragments of the olive tree (Olea europaea L.). Plant Sci 54:65–74
Capelo AM, Silva S, Brito G, Santos C (2010) Somatic embryogenesis induction in leaves and petioles of a mature wild olive. Plant Cell Tiss Organ Cult 103:237–242
Clavero-Ramírez I, Pliego-Alfaro F (1990) Germinación in vitro de embriones maduros de olivo (Olea europaea). Actas de Horticultura 1:512–516
Etienne H, Bertrand B (2003) Somaclonal variation in Coffea arabica: effects of genotype and embryogenic cell suspension age on frequency and phenotype of variants. Tree Physiol 23:419–426
FAOSTAT (2009) http://faostat.fao.org/
Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tiss Organ Cult 74:201–228
Finkelstein RR, Tenbarge KM, Shumway JE, Crouch ML (1985) Role of ABA in maturation of rapeseed embryos. Plant Physiol 78:630–636
Jiménez VM (2005) Involvement of plant hormones and plant growth regulators on in vitro somatic embryogenesis. Plant Growth Regulat 47:91–110
Krajňáková J, Häggman H, Gömöry D (2009) Effect of sucrose concentration, polyethylene glycol and activated charcoal on maturation and regeneration of Abies cephalonica somatic embryos. Plant Cell Tiss Organ Cult 96:251–262
Litz RE, Witjaksono, Raharjo S, Efendi D, Pliego-Alfaro F, Barceló-Muñoz A (2005) Persea americana avocado. In: Litz RE (ed) Biotechnology of Fruit and Nut Crops. CABI, Wallingford, Oxfordshire, pp 326–347
Maalej M, Drira N, Chaari-Rkhis A, Trigui A (2002) Preliminary results of somatic embryogenesis from young zygotic embryos of olive tree. Acta Hort 586:899–902
Mitrakos K, Alexaki A, Papadimitriou P (1992) Dependence of olive morphogenesis on callus origin and age. J Plant Physiol 139:269–273
Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15:473–497
Niedz RP, Hyndman SE, Wynn ET, Bausher MG (2002) Normalizing sweet orange (C. sinensis (L.) Osbeck) somatic embryogenesis with semi-permeable membranes. In Vitro Cell Dev Biol Plant 38:552–557
Orinos Th, Mitrakos K (1991) Rhizogenesis and somatic embryogenesis in calli from wild olive (Olea europaea var. sylvestris (Miller) Lehr) mature zygotic embryos. Plant Cell Tiss Organ Cult 27:183–187
Pérez-Barranco G, Torreblanca R, Padilla IMG, Sánchez-Romero C, Pliego-Alfaro F, Mercado JA (2009) Studies on genetic transformation of olive (Olea europaea L.) somatic embryos: I. Evaluation of different aminoglycoside antibiotics for nptII selection. II. Transient transformation via particle bombardment. Plant Cell Tiss Organ Cult 97:243–251
Prewein C, Vagner M, Wilhelm E (2004) Changes in water status and proline and abscisic acid concentrations in developing somatic embryos of pedunculate oak (Quercus robur) during maturation and germination. Tree Physiol 24:1251–1257
Quiroz-Figueroa FR, Rojas-Herrera R, Galaz-Avalos RM, Loyola-Vargas VM (2006) Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tiss Organ Cult 86:285–301
Revilla MA, Pacheco J, Casares A, Rodríguez R (1996) In vitro reinvigoration of mature olive trees (Olea europaea L.) through micrografting. In Vitro Cell Dev Biol Plant 32:257–261
Rugini E (1988) Somatic embryogenesis and plant regeneration in olive (Olea europaea L.). Plant Cell Tiss Organ Cult 14:207–214
Rugini E, Baldoni L (2005) Olea europaea Olive. In: Litz RE (ed) Biotechnology of fruit and nut crops. CABI, Wallingford, Oxfordshire, pp 404–428
Rugini E, Caricato G (1995) Somatic embryogenesis and plant recovery from mature tissues of olive cultivars (Olea europaea L.) ‘Canino’ and ‘Moraiolo’. Plant Cell Rep 14:257–260
Rugini E, Mencuccini M, Biasi R, Altamura MM (2005) Olive (Olea europea L.). In: Jain SM, Gupta PK (eds) Protocol for somatic embryogenesis in woody plants. Springer, Dordrecht, pp 345–360
Schenk RU, Hildebrand AC (1972) Medium and technique for induction and growth of monocotyledoneous and dicotyledoneous plant cell cultures. Can J Bot 50:199–204
Shibli RA, Shatnawi M, Abu-Ein Al-Juboory KH (2001) Somatic embryogenesis and plant recovery from callus of ‘Nabali’ olive (Olea europaea L.). Sci Hortic 88:243–256
Sokal RR, Rohlf FJ (1995) Biometry. W.H. Freeman, New York
Torreblanca R, Cerezo S, Palomo-Ríos E, Mercado JA, Pliego-Alfaro F (2010) Development of a high throughput system for genetic transformation of olive (Olea europaea L.) plants. Plant Cell Tiss Organ Cult 103:61–69
Trabelsi EB, Bouzid S, Bouzid M, Elloumi N, Belfeleh Z, Benabdallah A, Ghezel R (2003) In vitro regeneration of olive tree by somatic embryogenesis. J Plant Biol 46:173–180
Troch V, Werbrouck S, Geelen D, Van Labeke M-C (2009) Optimization of horse chestnut (Aesculus hippocastanum L.) somatic embryo conversion. Plant Cell Tiss Organ Cult 98:115–123
Von Arnold S (2008) Somatic embryogenesis. In: George EF, Hall MA, De Klerk G-J (eds) Plant propagation by tissue culture, vol. 1, The Background, 3rd edn. Springer, Dordrecht, pp 335–354
Acknowledgments
This research was funded by Dirección General de Investigación y Formación Agraria y Pesquera, Consejería de Agricultura y Pesca, Junta de Andalucía (Project CAO00-018-C7-5) and Agencia Española de Cooperación Internacional para el Desarrollo (Project A/017856/08).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Cerezo, S., Mercado, J.A. & Pliego-Alfaro, F. An efficient regeneration system via somatic embryogenesis in olive. Plant Cell Tiss Organ Cult 106, 337–344 (2011). https://doi.org/10.1007/s11240-011-9926-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-011-9926-6