Abstract
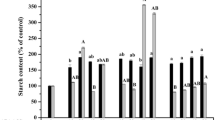
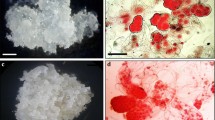
Low efficiency of embryo maturation, germination and conversion to plantlets is a major problem in many species including Persian walnut. We studied the effects of abscisic acid (ABA) and sucrose, on the maturation and germination of Persian walnut (Juglans regia) somatic embryos. Individual globular somatic embryos were grown on a maturation medium supplemented with different combinations of ABA and sucrose for ca. 1 month, until shoot meristems and radicles had developed. White and opaque embryos in late cotyledonary stage were subjected to desiccation after the culture period on maturation media. The number of germinated somatic embryos was influenced by the concentrations of ABA in the maturation medium. The best treatment for germination, in which both shoot and root were developed contained 2 mg l−1 ABA and resulted in 41% conversion of embryos into plantlets. Regeneration was reduced at higher levels of ABA. While ABA always reduced the rate of secondary embryogenesis, treatments containing 4.0% sucrose significantly increased the number of secondary embryos. On the other hand, sucrose had little influence on maturation. Normal and abnormal embryos were verified anatomically.





Similar content being viewed by others
Abbreviations
- ABA:
-
Abscisic acid
- DKW:
-
Driver & Kuniyuki walnut medium
- GA3 :
-
Gibberrelic acid
- SE:
-
Somatic embryogenesis
References
Ammirato PV (1989) Recent progress in somatic embryogenesis. Int Assoc Plant Tissue Cult Newsl 57:2–16
Ammirato PV (1997) Hormonal control of somatic embryo development from cultured cells of caraway. Interaction of abscisic acid, zeatin and gibberellic acid. Plant Physiol 59:579–586
Attree SM, Fowke LC (1993) Embryogeny of gymnosperms: advances in synthetic seed technology of conifers. Plant Cell Tissue Organ Cult 35:1–35
Cornu D (1988) Somatic embryogenesis in tissue culture of walnut (Juglans regia, J. major and hybrids J. nigra × Juglans regia). In: Ahuja MR (ed) Somatic cell genetics of woody plants. Kluwer Academic Publishers, Boston
Cornu D (1989) Walnut somatic embryogenesis, physiological and histological aspects. Ann Sci For 46S:133–135
Cornu D, Jay-Allemand C (1989) Micropropagation of hybrid walnut trees (Juglans nigra × Juglans regia) through culture and multiplication of embryos. Ann Sci For 46S:113–135
Deng MD, Cornu D (1992) Maturation and germination of walnut somatic embryos. Plant Cell Tissue Organ Cult 28:195–202
Dhuria HS, Bhutani VP, Dhar RP (1977) Standardization of propagation techniques in walnut. I. Preliminary studies on grafting. Indian J Hortic 34:20–23
Driver JA, Kuniyuki AH (1984) In-vitro propagation of paradox walnut rootstock. HortScience 19:507–509
Etienne H, Montoro P, Michaux-Ferriere N, Carron MP (1993) Effect of desiccation, medium osmolarity and abscisic acid of the maturation of Hevea brasiliensis somatic embryos. J Exp Bot 44:1613–1619
FAO (2005) FAO statistics division 2007. http://faostat.fao.org. Cited 13 February 2007
Gutmann M, Aderkas PV, Label P, Lelu MA (1996) Effects of abscisic acid on somatic embryo maturation of hybrid larch. J Exp Bot 47:1905–1917
Harry IS, Thorpe TA (1991) Somatic embryogenesis and plant regeneration from mature zygotic embryos of red spruce. Bot Gaz 152:446–452
Hartmann HT, Kester DE, Davies FT, Geneve RL (1997) Plant propagation: principles and practices, 6th edn. Prentice Hall Int’l, INC
Komatsuda T, Lee W, Oka S (1992) Maturation and germination of somatic embryos as affected by sucrose and plant growth regulators in soybeans Glycine gracilis Skvortz and Glycine max (L.) Merr. Plant Cell Tissue Organ Cult 28:103–113
Kuden A, Kaska N (1997) Studies on the patch-budding of walnuts in different budding periods under subtropical conditions. Acta Hortic 442:299–301
Lee BC, Shim SY, Lee SK (1988) Mass propagation and germination of somatic embryos in Juglans regia L. (English walnut). Res Rep Inst For Genet Korea 24:99–106
Long LM, Preece JE, Van Sambeek JW (1995) Adventitious regeneration of Juglans nigra L. (eastern black walnut). Plant Cell Rep 8:512–516
Mauri PV, Manzanera JA (2003) Induction, maturation and germination of Holm oak (Quercus ilex L.) somatic embryos. Plant Cell Tissue Organ Cult 74:229–235
McGranahan G, Leslie C, Uratsu S, Dandekar A (1990) Improved efficiency of the walnut somatic embryo gene transfer system. Plant Cell Rep 8:512–516
Misra S (1994) Conifer zygotic embryogenesis, somatic embryogenesis, and seed germination: biochemical and molecular advances. Seed Sci Res 4:357–384
Nuutila AM, Kurten U, Kauppinen V (1991) Optimization of sucrose and inorganic nitrogen concentrations for somatic embryogenesis of birch (Betula pendula Roch.) callus cultures: a statistical approach. Plant Cell Tissue Organ Cult 24:73–77
Ozkan Y, Gumus A (2001) Effect of different applications on grafting under controlled conditions of walnut (Juglans regia L.). Acta Hortic 544:515–520
Ozkan Y, Edizer Y, Akca Y (2000) A study in propagation with patch budding of walnut cultivars (J. regia L.). Acta Hortic 544:521–525
Park YS, Lelu-Walter MA, Harvengt L, Trontin JF, MacEacheron I, Klimaszewska K, Bonga JM (2006) Initiation of somatic embryogenesis in Pinus banksiana, P. strobus, P. pinaster and P. sylvestris at three laboratories in Canada and France. Plant Cell Tissue Organ Cult 86:87–101
Pond SE, Von Aderkas P, Bonga JM (2002) Improving tolerance of somatic embryos of Picea glauca to flash desiccation with a cold treatment (desiccation after cold acclimation). In Vitro Cell Dev Biol Plant 38:334–341
Preece JE, McGranahan GH, Long LM, Leslie CA (1995) Somatic embryogenesis in walnut (Juglans regia). In: Jain S, Gupta P, Newton R (eds) Somatic embryogenesis in woody plants, vol 2. Kluwer Academic Publishers, Netherlands
Rezaee R, Vahdati K, Grigoorian W, Valizadeh M (2008) Walnut grafting success and bleeding rate as affected by different grafting methods and seedling vigor. J Hortic Sci Biotechnol 83(1):94–99
Roberts-Oehlschlager SL, Dunwell JM, Faulks R (1990) Changes in the sugar content of barley anthers during culture of different carbohydrates. Plant Cell Tissue Organ Cult 22:77–85
Roghani MS, Haq A, Khan J (1977) Propagation of walnut by grafting. J Agric Res 14:389–392
SAS institute Inc (1985) SAS® user’s guide: statistics. Version 5 Edition. SAS institute Inc, Cary
Tang H, Ren Z, Krczal G (2000) Improvement of English walnut somatic embryo germination and conversion by desiccation treatment and plantlet development by lower medium salts. In Vitro Cell Dev Biol Plant 36:47–50
Tang H, Ren Z, Reustle G, Krczal G (2001) Optimizing secondary somatic embryo production in English walnut (Juglans regia L.). Acta Hortic 544:187–194
Tian L, Brown D (2000) Improvement of soybean somatic embryo development and maturation by abscisic acid treatment. Can J Plant Sci 80:271–276
Trembly L, Trembly F (1995) Maturation of black spruce somatic embryos: sucrose hydrolysis and resulting osmotic pressure of the medium. Plant Cell Tissue Organ Cult 42:39–46
Tulecke W (1987) Somatic embryogenesis in woody perennials. In: Bonga JM, Durzan DJ (eds) Cell tissue cult forestry, vol 2. Martinus Nijhoff Publ, Boston
Tulecke W, McGranahan G (1985) Somatic embryogenesis and plant regeneration from cotyledons of walnut, Juglans regia L. Plant Sci 40:57–63
Tulecke W, McGranahan G, Ahmadi H (1988) Regeneration by somatic embryogenesis of triploid plants from endosperm of walnut, Juglans regia L. cv. ‘Manregian’. Plant Cell Rep 7:301–304
Vahdati K (2001) Walnut situation in Iran. Nucis-Newsl 9:32–33
Vahdati K, McKenna J, Dandekar A, Leslie C, Uratsu S, Hackett W, Negri P, McGranahan G (2002) Rooting and other characteristics of a transgenic walnut hybrid (Juglans hindsii × J. regia) rootstock expressing rolABC. J Am Soc Hortic Sci 127:724–728
Vahdati K, Leslie C, Zamani Z, McGranahan G (2004) Rooting and acclimatization of in-vitro grown shoots from three mature Persian walnut cultivars. HortScience 39:324–327
Vahdati K, Jariteh M, Niknam V, Mirmasoumi M, Ebrahimzadeh H (2006) Somatic embryogenesis and embryo maturation in Persian walnut. Acta Hortic 705:199–205
Von Arnold S, Sabala I, Bozhkov P, Dyachok J, Filonova L (2002) Developmental pathways of somatic embryogenesis. Plant Cell Tissue Organ Cult 69:233–249
Acknowledgments
The authors thank dean assistance of research of College of Abooraihan, University of Tehran and Iranian National Science Foundation (INSF) for providing financial supports for this research. Dr. Ebrahim Firoozabady and Dr. Alireza Nabipour are also acknowledged for pre-reviewing the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Vahdati, K., Bayat, S., Ebrahimzadeh, H. et al. Effect of exogenous ABA on somatic embryo maturation and germination in Persian walnut (Juglans regia L.). Plant Cell Tiss Organ Cult 93, 163–171 (2008). https://doi.org/10.1007/s11240-008-9355-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11240-008-9355-3