Abstract
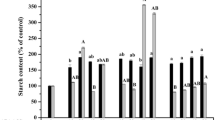
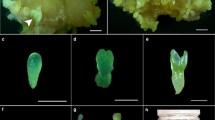
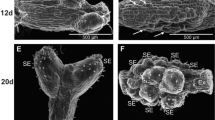
The physiological and osmotic roles of sucrose during black spruce (Picea mariana (Mill.) B.S.P.) embryo maturation were investigated. The results showed that when both sucrose and mannitol were present in the medium, the optimum sucrose concentration varied between 4% and 6%. From these data, mannitol does not apparently replace sucrose during the maturation of somatic embryos and therefore it might not be a suitable osmoticum. For the media supplemented with 4% to 12% sucrose and various concentrations of mannitol, the osmotic pressure of the medium rose during maturation, particularly for the highest sucrose concentrations (7% to 12%). Medium containing 3% each of fructose and glucose produced fewer mature embryos compared to the medium with 6% sucrose. An increment in the osmotic potential was observed in medium with 6% sucrose in contrast to that containing 3% each of fructose and glucose. Sugar analysis revealed that the sucrose hydrolysis in the medium was detectable within 1 week of incubation and continued throughout the maturation period. Moreover, no significant uptake of the sugars was detected, since the total amount of fructose, glucose and sucrose remained constant. Our results indicate that the action of sucrose on embryo maturation is mostly achieved through an osmotic control.
Similar content being viewed by others
References
Anandarajah K & McKersie BD (1990) Manipulating the desiccation tolerance and vigor of dry somatic embryos of Medicago sativa L. with sucrose, heat shock and abscisic acid. Plant Cell Rep. 9: 451–455
Attree SM, Moore D, Sawhney VK & Fowke LC (1991) Enhanced maturation and desiccation tolerance of white spruce (Picea glauca (Moench) Voss) somatic embryos — Effects of a nonplasmolysing water stress and abscisic acid. Ann. Bot. 68: 519–525
Attree SM, Pomeroy MK & Fowke LC (1992) Manipulation of conditions for the culture of somatic embryos of white spruce for improved triacylglycerol biosynthesis and desiccation tolerance. Planta 187: 395–404
Cho B-H & Kornor E (1985) Comparison of suspension cells and cotyledons of Ricinus with respect to sugar uptake. J. Plant Physiol. 118: 381–390
Cram WJ (1984) Mannitol transport and suitability as an osmoticum in root cells. Physiol. Plant. 61: 396–404
Davidson JG (1990) La nécessité est la mère de l'invention. Info-Forêts 9: 2–3
Desjardins Y, Hdider C & de Rick J (1994) Carbon nutrition in vitro — Regulation and manipulation of carbon assimilation in micropropagated systems. In: Aitken-Christie J, Kozai T & Smith ML (Eds) Automation and Environmental Control in Plant Tissue Culture (pp 441–471) Kluwer Academic Publishers, Dordrecht
Dracup M, Gibbs J & Greenway H (1986) Melibiose, a suitable nonpermeating osmoticum for suspension-cultured tobacco cells. J. Exp. Bot. 37: 179–189
Finer JJ, Kriebel HB & Becwar MR (1989) Initiation of embryogenic callus and suspension cultures of eastern white pine (Pinus strobus L.). Plant Cell Rep. 8: 203–206
Kononowicz AK & Janick J (1984) The influence of carbon source on the growth and development of asexual embryos of Theobroma cacao. Physiol. Plant. 61: 155–162
Krizek DT (1985) Methods of inducing water stress in plants. HortScience 20: 1028–1034
Litvay JD, Verma DC & Johnson MA (1985) Influence of loblolly pine (Pinus taeda L.) culture medium and its components on growth and somatic embryogenesis of the wild carrot (Daucus carota L.). Plant Cell Rep. 4: 325–328
Maretzki A & Thom M (1972) Membrane transport of sugar in cell suspensions of sugarcane. I. Evidence for sites and specificity. Plant Physiol. 49: 177–182
Maretzki A, Thom M & Nickell LG (1974) Utilization and metabolism of carbohydrates in cell and callus cultures. In: Street HE (Ed) Tissue Culture and Plant Science (pp 329–361) Academic Press, London
Misra S, Attree SM, Leal I & Fowke LC (1993) Effect of abscisic acid, osmoticum, and desiccation on synthesis of storage proteins during the development of white spruce somatic embryos. Ann. Bot. 71: 11–22
Morris P & Fowler MW (1980) Sucrose utilization by cell suspension cultures of Catharanthus roseus G. Don. Biochem. Soc. T. 8: 630–631
Nadel BL, Altman A & Ziv M (1989) Rogulation of somatic embryogenesis in celery cell suspensions. I. Promoting effects of mannitol on somatic embryo development. Plant Cell Tiss. Org. Cult. 18: 181–189
Newton RJ, Puryear JD, Bhaskaran S & Smith RH (1990) Polyethylene glycol content of osmotically stressed callus cultures. J. Plant Physiol. 135: 646–652
Roberts DR (1991) Abscisic acid and mannitol promote early development, maturation and storage protein accumulation in somatic embryos of interior spruce. Physiol. Plant. 83: 247–254
St-Laurent L, Bousquet J, Simon L & Lalonde M (1987) Separation of various Frankia strains in the Alnus and Elaeagnus host specificity groups using sugar analysis. Can. J. Microbiol. 33: 764–772
Steel RGD & Torrie JH (1980) Principles and Procedures of Statistics: A Biometrical Approach. 2nd edition, McGraw-Hill Book Company, New York, 633 p
Thompson MR & Thorpe TA (1987) Metabolic and non-metabolic roles of carbohydrates. In: Bonga JM & Durzan DJ (Eds) Cell and Tissue Culture in Forestry, Vol 1 (pp 89–112) Martinus Nijhoff Publishers, Dordrecht
Thompson MR, Douglas TJ, Obata-Sasamoto H & Thorpe TA (1986) Mannitol metabolism in cultured plant cells. Physiol. Plant. 67: 365–369
Thorpe TA (1974) Carbohydrate availability and shoot formation in tobacco callus cultures. Physiol. Plant. 30: 77–81
Thorpe TA (1982) Carbohydrate utilization and metabolism. In: Bonga JM & Durzan DJ (Eds) Tissue Culture in Forestry (pp 325–368) Martinus Nijhoff Publishers, Dordrecht
Thorpe TA & Meier DD (1973) Sucrose metabolism during tobacco callus growth. Phytochemistry 12: 493–497
Treat WJ, Engler CR & Soltes EJ (1989) Culture of photomixotropic soybean and pine in a modified fermentor using a novel impeller. Biotechnol. Bioeng. 34: 1191–1202
Tremblay FM (1990) Somatic embryogenesis and plantlet regeneration from embryos isolated from stored seeds of Picea glauca. Can. J. Bot. 68: 236–242
Tremblay L & Tremblay FM (1991a) Effects of gelling agents, ammonium nitrate concentration, and light on the development of Picea mariana (Mill.) B.S.P. (black spruce) and P. rubens Sarg. (red spruce) somatic embryos. Plant Sci. 77: 233–242
Tremblay L & Tremblay FM (1991b) Carbohydrate requirements for the development of black spruce (Picea mariana (Mill.) B.S.P.) and red spruce (P. rubens Sarg.) somatic embryos. Plant Cell Tiss. Org. Cult. 27: 95–103
Tremblay L & Tremblay FM (1995) Somatic embryogenesis in black spruce (Picea mariana (Mill.) B.S.P.) and red spruce (P. rubens Sarg.). In: Bajaj YPS (Ed), Biotechnology in Agriculture and Forestry: Somatic embryogenesis and synthetic seed I, Vol. 30, Springer-Verlag, Berlin, pp. 431–443
Trip P, Krotkov G & Nelson CD (1964) Metabolism of mannitol in higher plants. Amer. J. Bot. 51: 828–835
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Tremblay, L., Tremblay, F.M. Maturation of black spruce somatic embryos: Sucrose hydrolysis and resulting osmotic pressure of the medium. Plant Cell Tiss Organ Cult 42, 39–46 (1995). https://doi.org/10.1007/BF00037680
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00037680