Abstract
The aim of this study was to investigate whether ABA, sucrose or Phytagel applied to the maturation medium at different concentrations affected the growth, development and starch content of somatic embryos of Picea abies and Picea omorika. Embryogenic tissues of both spruce species were placed on maturation medium supplemented with various doses of ABA (10, 20, 40 and 80 µM), sucrose (17, 34 and 68 g/L) or Phytagel (4, 6 and 8 g/L). Our results showed that ABA and the osmoticum had a significant effect on the production and maturation of somatic embryos of both spruce species. Supplementing the medium with low concentrations of ABA (10 µM) or sucrose (17 g/L) resulted in the precocious germination of embryos. Adding sucrose to the maturation medium at the highest tested concentration (68 g/L) improved the growth of the radicles of the embryos of both spruce species during the germination stage. Moreover, the intensity of the growth of the hypocotyls and radicles of P. omorika germinating embryos depended on the Phytagel concentration applied to the maturation medium. The starch content and the starch accumulation pattern of Picea abies and P. omorika somatic embryos were dependent on the concentration of ABA, sucrose or Phytagel in the maturation medium. In general, during development, the P. abies somatic embryos accumulated a much greater amount of starch than P. omorika embryos did. The effects of ABA and osmoticum on the starch accumulation pattern of Picea abies and P. omorika somatic embryos are first reported in this paper.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Somatic embryogenesis is the most useful micropropagation method for conifers (Bonga et al. 2010). Plant breeding using the somatic embryogenesis technique is a multistep process that requires the development of efficient protocols for each developmental stage of the somatic embryos (Garin et al. 2000; Hogberg et al. 2001; Szczygieł et al. 2007; Hazubska-Przybył et al. 2008; Lelu-Walter and Pâques 2009; Deo et al. 2010). The fundamental problems associated with this propagation method are the following: the loss of the embryogenic potential of the obtained cultured lines shortly after their establishment, the proper maturation of the somatic embryos and their limited ability for germination, conversion into plants and adaptability to natural conditions (Hazubska and Szczygieł 2003; Szczygieł et al. 2007; Lelu-Walter and Pâques 2009). Moreover, the somatic embryogenesis process of conifers is often highly genotype dependent, and the development of universal propagation protocols is associated with several difficulties (Kong and von Aderkas 2007; Hazubska-Przybył and Bojarczuk 2008; Bonga et al. 2010; Teixteira da Silva and Malabadi 2012). Only by overcoming these difficulties can the somatic embryogenesis method be used more widely.
Many investigations of the somatic embryogenesis of coniferous species showed that abscisic acid (ABA), osmotically active agents and gelling agents significantly impacted the maturation and germination of somatic embryos (Hakman and von Arnold 1988; Gutmann et al. 1996; Teyssier et al. 2011; Teixteira da Silva and Malabadi 2012). ABA and osmotic stress are the key factors that determine the proper maturation of somatic embryos. Both factors also play an important role in the accumulation of reserve materials, such as proteins, lipids and carbohydrates, in the developing embryos and in the synchronisation of their development during the maturation stage (Misra 1994; von Aderkas et al. 2002; Kermode 2005; Businge et al. 2013). Moreover, ABA promotes the development of somatic embryos from embryogenic tissues, and during their maturation stage, ABA triggers and enhances their tolerance to desiccation and inhibits precocious germination (Kermode 2005; Rai et al. 2011).
Sucrose is one of the osmotically active agents that is most frequently used during the maturation of conifer somatic embryos. Sucrose is a source of carbon and energy and may participate in regulating the expression of genes that affect the embryonic maturation process (Lipavská et al. 2000; Iraqi and Tremblay 2001; Iraqi et al. 2005). This compound is often added to the culture medium to improve the maturation rate of somatic embryos (Lema-Rucińska et al. 2013) and to increase the content of storage materials, such as starch and oligosaccharides, in the embryos (Kępczyńska 2006).
Sucrose participates in many of the metabolic processes of plants. Sucrose is synthesised in source tissues and is transported to sink tissues, where it is stored or utilised (Sauer 2007; Wind et al. 2010). Changes in the levels of synthesis, transport and degradation of sucrose affect the growth, development and physiology of plants (Wind et al. 2010).
Phytagel is the gelling agent that is most commonly applied in conifer embryogenic cultures (Kim et al. 1999; Klimaszewska et al. 2000). Some studies showed that supplementing culture media with Phytagel at the appropriate concentration had a positive effect on the development and growth of somatic embryos of certain coniferous species (Garin et al. 2000; Klimaszewska et al. 2000; Lelu-Walter and Pâques 2009; Teyssier et al. 2011; Morel et al. 2014).
During plant embryogenesis, reserve materials, including starch, are accumulated (Kermode 2005). The process of starch accumulation in the form of characteristic grains during the somatic embryogenesis of conifers has been described (Misra 1994; Iraqi and Tremblay 2001). This process was particularly intense after the embryogenic tissue was placed on maturation medium supplemented with ABA (Misra 1994). Starch grains began to appear in the cells of early, immature somatic embryos, and their content changed as embryonic development progressed (Gutmann et al. 1996). At the beginning of development, the number and size of the starch grains increased, whereas during the cotyledonary stage of development, the starch content gradually decreased as it was mobilised by the growing embryo (Salopek et al. 1997; Hazubska-Przybył et al. 2008).
P. abies and P. omorika occur naturally in Europe. Both of these spruce species are often planted in parks and gardens because of their decorative qualities. Between the mid-1980s and the early 1990s, the successful application of the somatic embryogenesis method for the micropropagation of both spruce species was achieved (Hakman et al. 1985; Chalupa 1985; Budimir and Vujičić 1992). Thereafter, P. abies became the model species for the study of various aspects of conifer somatic embryogenesis (Hakman and von Arnold 1988; Lipavská et al. 2000; Malá et al. 2009; Sun et al. 2011; Bříza et al. 2013; Hazubska-Przybył et al. 2013). Few studies have investigated the somatic embryogenesis of P. omorika (Tramisak-Milaković et al. 1999; Leljak-Levanić et al. 2009; Hazubska-Przybył et al. 2010), a more unique spruce species because of its endemic and relict character (Vujičić and Budimir 1995; Ballian et al. 2006). To date, the results of research on the propagation of both spruce species via the somatic embryogenesis technique are not entirely satisfactory; thus, this method cannot be applied on a wider scale. Therefore, there is a need to develop efficient protocols for the most highly productive in vitro micropropagation of P. abies and P. omorika using this technique.
The aim of this study was to determine the effect of ABA, sucrose and Phytagel at various concentrations on the growth, development and starch content of Picea abies and P. omorika embryos obtained via somatic embryogenesis.
Materials and methods
Embryogenic tissue origin
The somatic embryogenesis of mature zygotic embryos taken from seeds of Picea abies and P. omorika that were collected from trees growing in the Experimental Forest ‘Zwierzyniec’ near Kórnik (provenance Serwy) and in the Kórnik Arboretum (52º15′N, 17º04′E), respectively, was induced. The zygotic embryos (explants) were cultured on half-strength LM medium (½ LM; Litvay et al. 1985) supplemented with 9 µM 2,4-D and 8.8 µM BA for 8 weeks to induce the production of embryogenic tissues (ETs). Subsequently, the ETs were allowed to proliferate on medium supplemented with 9 µM Picloram and 4.5 µM BA.
Maturation of the somatic embryos
To obtain somatic embryos, pieces of actively growing Picea abies and P. omorika ETs were placed on growth regulator-free ½ LM medium supplemented with 1 % activated charcoal (Sigma) and 34 g/L sucrose and were incubated for 7 days. Next, the ETs of both of the spruce species were transferred to maturation medium containing different concentrations of abscisic acid (ABA), sucrose or Phytagel and were incubated for 7 weeks.
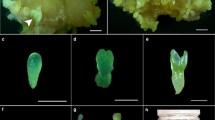
During the first 3 weeks somatic embryos exhibited precotyledonary (from globulary to torpedo) stage. In the 5th week embryos started to reach the cotyledonary stage. In the next 2 weeks they continued their growth entering late-cotyledons stage. C refers simply to cotyledonary stage. Somatic embryos at late-cotyledons stage were taken to begin the plantlet conversion. Each developmental stage is supported by a representative picture (Supplemental Fig. 1).
Experiment I: effect of abscisic acid
Embryogenic tissues of Picea abies and P. omorika were placed on medium containing IBA (1 µM), sucrose (34 g/L), Phytagel (6 g/L) and ABA at different concentrations, namely, 10, 20, 40 and 80 µM. This experiment was repeated three times for Picea abies and two times for P. omorika. Three replicates were used per each treatment.
Experiment II: effect of sucrose
Embryogenic tissues of both spruce species were placed on medium containing IBA (1 µM), ABA (20 µM), Phytagel (6 g/L) and sucrose at different concentrations, namely, 17, 34 and 68 g/L. This experiment was repeated three times for Picea abies and P. omorika. Three replicates were used per each treatment.
Experiment III: effect of phytagel
Embryogenic tissues of Picea abies and P. omorika were placed on medium containing IBA (1 µM), ABA (20 µM) and sucrose (34 g/L) and Phytagel at different concentrations, namely, 4, 6, 8 g/L. This experiment was repeated two times for Picea abies and three times for P. omorika. Three replicates were used per each treatment.
The pH of the media was adjusted to 5.8 prior to autoclaving at 121 °C and 100 kPa for 20 min. The growth regulators (ABA and IBA-indole-3-butyric acid) and l-glutamine were filter-sterilised and were added to the media after autoclaving.
ETs were dispersed on Whatman number 2 filter papers, which were placed on the maturation media. The cultures were placed under blue and red LED light (light intensity of 35 µM m−2 s−1; 16-h photoperiod) at 22 ± 1 °C. After 5 weeks, all produced somatic embryos from globular to cotyledonary stage (P) and cotyledonary somatic embryos (C) per 1 g of embryogenic tissue was determined. Finally, the percentage of somatic embryos capable of maturation was assessed. Three Petri dishes each (90-mm diameter) containing 200 mg of embryogenic tissue were used per variant.
Analysis of starch content
The starch content of ETs (zero treatment) was determined before transferring them to maturation medium and that of the somatic embryos was determined at the precotyledonary (3 weeks of cultivation on maturation medium), cotyledonary (5 weeks of cultivation) and late-cotyledonary (7 weeks of cultivation) stages. The latter embryos had the ability to conduct photosynthesis due to the presence of chlorophyll. Somatic embryos subjected to each treatment were collected for analysis.
The starch content was determined according to the method of Huber and Israel (1982). ETs and somatic embryos were both extracted using 80 % ethanol. The pellet obtained by centrifugation (at 4 °C, 15,000g for 20 min) was suspended in 2 ml of 0.2 N KOH and placed in boiling water for 30 min. After cooling, the pH of the mixture was adjusted to 5.5 using 1 M acetic acid. An equal volume of a solution of amyloglucosidase (400 units/ml in 9.1 M citrate buffer, pH 5.5) was added. After an incubation (at 45 °C for 4 h), the glucose concentration of the supernatant was determined according to the modified method of Somogyi (1952).
Germination of the somatic embryos
Cotyledonary somatic embryos of each variant of both of the tested spruce species obtained in three above-described experiments were removed from the ½ LM at the 5th week of culture and were transferred to ME medium (Margara 1977) lacking growth regulators but supplemented with sucrose (20 g/L) to allow germination. They were cultured for 2 weeks in darkness (first phase) and then, for the next 2 weeks, in blue and red LED light (second phase; light intensity and photoperiod as above) at 22 ± 1 °C. After each phase, the length of the hypocotyls and radicles was measured to determine the germination capacity of the somatic embryos. Three Petri dishes containing 60 embryos per variant were used to test the germination capacity. Each experiment was repeated twice. Somatic embryos were treated as germinated when the radical started to protrude (Kępczyńska 2006).
Statistical analysis
The number of produced (P) and cotyledonary (C) somatic embryos and the percentage of mature embryos (calculated as the number of cotyledonary embryos/all produced embryos on the maturation medium × 100) were analysed using a one-way ANOVA and a post hoc Tukey test (p < 0.05). Bliss transformation of the maturation frequency data was performed before the statistical analysis was conducted. The data concerning the germination of somatic embryos and the starch content were analysed using the Kruskal–Wallis and Dunn’s tests (p < 0.05). The homogeneity of the variances was verified using Levene’s test (STATISTICA software, StatSoft Polska, Kraków, 1995–2005).
Results
Maturation of the somatic embryos
Experiment I: effect of abscisic acid
The ABA concentration in the maturation medium had no effect on the production of precotyledonary somatic embryos of P. abies. In contrast, the ABA concentration affected the number of P. abies embryos observed at the cotyledonary (C) stage (Table 1). The application of 40 µM ABA was most beneficial for the formation of embryos at the cotyledonary stage. In the case of P. omorika, the ABA concentration had a significant effect on both the production and maturation of somatic embryos. The least favourable concentration of ABA was 40 µM. The most effective results were obtained using 10 µM ABA (78 P/g ET and 50 C/g ET).
ABA treatment during the maturation period affected the ability of the immature somatic embryos of P. abies to reach the cotyledonary stage. The highest maturation frequency (60 %) accompanied by the highest number of produced (506 P/g ET) and cotyledonary-stage (327 C/g ET) embryos was observed when 40 µM ABA was applied (Table 1). Significant differences in the maturation frequency of the P. omorika embryos were also observed. The best results (64 %) were obtained using the lowest concentration of ABA (10 µM) (Table 1). However, during the experiment, we observed that the somatic embryos of both spruce species showed a tendency to precocious germination when matured on the lowest dose of ABA (data not shown). Increasing the dose of ABA to 20 µM (Control) inhibited the precocious germination of the somatic embryos and led to a slight decrease in the number of somatic embryos that reached the cotyledonary stage of development (Table 1).
Experiment II: effect of sucrose
The presence of sucrose in the maturation medium at concentrations of 17, 34 or 68 g/L significantly affected the number of obtained somatic embryos of both spruce species (Table 2). The highest number of P. abies somatic embryos (463 P/g ET) was obtained from ETs cultured in the presence of sucrose at 34 g/L. Additionally, the highest number of cotyledonary-stage embryos (185 C/g ET) was observed on this medium. The sucrose concentration in the maturation medium did not affect the ability of P. abies somatic embryos to undergo maturation, with the average maturation frequency being approximately 43 % independent of the sucrose concentration. In contrast, the highest number of P. omorika somatic embryos (272 P/g ET and 220 C/g ET) was observed upon applying sucrose at 17 g/L. Furthermore, in this spruce species, the sucrose concentration had a significant effect on the maturation frequency (Table 2). The highest maturation frequency (81 %) was observed when 17 g/L sucrose was used, and the lowest maturation frequency (49 %) was observed when sucrose was used at 68 g/L. Unfortunately, the somatic embryos that matured on medium containing the lowest concentration of sucrose (17 g/L) had a tendency towards precocious germination. This concentration of sucrose also proved to be unfavourable for the proper development of P. abies somatic embryos, which germinated too early.
Experiment III: effect of Phytagel
The application of Phytagel at three different concentrations had a significant effect on the ability of P. abies ETs to produce somatic embryos (Table 3). The highest number of P. abies somatic embryos (430 P/g ET) was observed on maturation medium supplemented with Phytagel at 4 g/L, and the highest number of P. omorika somatic embryos (355 P/g ET) was observed on medium supplemented with Phytagel at 6 g/L. The number of cotyledonary somatic embryos produced was also the highest using these two concentrations of Phytagel. In both cases, the Phytagel dose had a significant effect on the number of cotyledonary somatic embryos. Significant differences in the maturation frequency of both spruce species were observed in the presence of different concentrations of Phytagel; the addition of 6 g/L Phytagel resulted in the highest maturation frequency.
Analysis of starch content
Experiment I: effect of abscisic acid
The concentration of ABA in the maturation medium significantly and differentially affected the starch accumulation patterns of somatic embryos of P. abies and P. omorika. The fluctuation in the starch content of maturing and mature P. abies embryos was milder than that of P. omorika embryos. The starch content of the majority of 3-, 5- and 7-week-old somatic embryos was nearly 2-fold higher than that of the embryogenic tissues (Fig. 1). It was not possible to determine one specific concentration of ABA that led to the highest rate of starch accumulation; however, the older were the P. omorika embryos, the higher was the ABA concentration needed for the acquisition of the highest starch content. The differences between the starch contents of embryos at different stages of their development that had been treated with ABA at various concentrations were small.
Starch content of embryogenic tissues (0 weeks of cultivation, not treated with ABA) and somatic embryos (3, 5 and 7 weeks of cultivation) of Picea abies and P. omorika during their maturation in the presence of ABA at different concentrations. Upper and lower case letters relate to P. omorika and P. abies, respectively. Bars are mean ± standard error (SE). Means followed by same letters in the bar are not significantly different (P = 0.05) using Kruskal–Wallis and Dunn’s tests
The pattern of starch accumulation in the somatic embryos of P. omorika was more dependent on the concentration of ABA in the medium. The precotyledonary-stage embryos had the highest starch content of the embryos treated with 20 µM ABA, which was more than 2-fold higher than that of the ETs (Fig. 1). The cotyledonary-stage somatic embryos had the highest starch content (more than 3-fold higher than that of the ETs) among the embryos treated with 40 µM ABA. In the late cotyledonary-stage embryos, the starch content was reduced and was slightly lower than that of the ETs. Finally, embryos treated with 10 µM ABA exhibited similar starch levels from 0 to 7 weeks of cultivation.
Experiment II: effect of sucrose
The concentration of sucrose applied to the maturation medium significantly affected the starch-accumulation ability of P. abies somatic embryos at 3 and 5 weeks of cultivation and of P. omorika somatic embryos at 5 and 7 weeks of cultivation (Fig. 2). Comparing two species at each treatment, P. abies somatic embryos accumulated significantly more starch than the P. omorika somatic embryos, with the exception of the 3-week-old embryos of both species, in which the starch contents were equivalent (Fig. 2). Greatly increased starch accumulation occurred in both P. abies and P. omorika embryos that matured in the presence of sucrose at a concentration of more than 17 g/L. The highest starch content, which was approximately 28-fold higher than that of the ETs, was observed in the cotyledonary-stage P. abies somatic embryos that had developed in the presence of 68 g/L of sucrose. In the late cotyledonary-stage embryos, this value was 1.5 times lower.
Starch content of embryogenic tissues (0 weeks of cultivation, not treated with sucrose) and somatic embryos (3, 5 and 7 weeks of cultivation) of Picea abies and P. omorika during maturation in the presence of sucrose at different concentrations. Upper and lower case letters relate to P. omorika and P. abies, respectively. Bars are mean ± standard error (SE). Means followed by same letters in the bar are not significantly different (P = 0.05) using Kruskal–Wallis and Dunn’s tests
Notable changes in the starch content were observed when the P. omorika somatic embryos were treated with sucrose at 68 g/L during maturation. At the 3rd week of culture, the starch content of the somatic embryos was 7-fold higher than that of the ETs, and it increased continuously up to the 7th week of culture (Fig. 2).
Experiment III: effect of phytagel
The pattern of starch accumulation in the 3-, 5- and 7-week-old somatic embryos of the two spruce species varied depending on the dose of Phytagel applied to the maturation medium. The starch content of the developing somatic embryos was significantly higher than that of the ETs (Fig. 3). In 3-week-old P. abies somatic embryos grown in the presence of Phytagel at 6 g/L, the starch content was found to be more than 3-fold higher than that of the ETs. In the following weeks, the starch content of these embryos decreased. However, in the embryos that were incubated on medium supplemented with Phytagel at 4 or 8 g/L, the starch content was further increased at the 5th week of culture and was decreased at the 7th week.
Starch content of embryogenic tissues (0 weeks of cultivation, not treated with Phytagel) and somatic embryos (3, 5 and 7 weeks of cultivation) of Picea abies and P. omorika during maturation in the presence of Phytagel at different concentrations. Upper and lower case letters relate to P. omorika and P. abies, respectively. Bars are mean ± standard error (SE). Means followed by same letters in the bar are not significantly different (P = 0.05) using Kruskal–Wallis and Dunn’s tests
P. omorika somatic embryos contained the greatest amount of starch at the precotyledonary stage (Fig. 3). Later, in the majority of the embryos, the starch content continuously decreased with development or did not change (7th week, 8 g/L Phytagel).
Germination of the somatic embryos
Experiment I: effect of abscisic acid
Germination of P. abies somatic embryos was conducted for 2 weeks in darkness (first phase) and for the next 2 weeks in light (second phase). The most intensive hypocotyl and radicle growth was observed in the presence of 80 µM ABA (Table 4). During the second phase of germination, the hypocotyls and radicles extended for 15.1 mm and 5.3 mm, respectively. The other ABA concentration did not significantly affect the growth of the hypocotyls and radicles of P. abies somatic embryos during either phase of germination. Similarly, the hypocotyl to radicle (H:R) length ratio was not significantly altered by the tested concentrations of ABA. The best synchronisation of hypocotyl and radicle growth was obtained using 20 µM ABA (Control-0), in which the H:R ratio was 2.6. For technical reasons, this experiment was not conducted using P. omorika somatic embryos.
Experiment II: effect of sucrose
Applying sucrose to the maturation medium proved to significantly affect the growth of the radicles, particularly during the second phase of germination of P. abies somatic embryos. The presence of sucrose at 68 g/L led to the radicles growing up to an average of 7.9 mm (Table 5). In contrast, the growth of the hypocotyls in each sucrose-dose treatment was comparable (13.5 mm on average). Somatic embryos matured in the presence of 68 g/L of sucrose were characterised by better synchronisation of the hypocotyl and radicle growth compared with that of embryos matured in the presence of lower concentrations of sucrose.
Significant differences in the radicle growth of germinating P. omorika somatic embryos were observed after growth in darkness and light. Embryos that matured on medium supplemented with 68 g/L of sucrose produced longer radicles during both phases of germination than did embryos treated with sucrose at lower concentrations (Table 5). The growth of somatic embryos treated with 34 g/L of sucrose was perfectly synchronised because the H:R ratio was 1.0. Applying sucrose at other concentrations led to much more intense growth of the hypocotyls than of the radicles.
Experiment III: effect of Phytagel
The Phytagel dose in the maturation medium did not significantly affect the dynamics of germination of P. abies somatic embryos (Table 6). Despite some differences in the growth of hypocotyls and radicles of individual variants during the first phase of germination of the somatic embryos, during the second phase, the growth of the hypocotyls and radicles was very similar regardless of the Phytagel dose applied. The approximately 3-fold faster growth rate of the hypocotyls led to poor growth synchronisation.
Differences between hypocotyl and radicle growth were observed during both phases of germination of the P. omorika embryos. Somatic embryos treated with 6 or 8 g/L of Phytagel produced longer radicles during the first phase of germination than did embryos treated with the lowest concentration of Phytagel, although the differences were not significant (Table 6). During the second phase of germination, the longest radicles were detected in embryos that had matured in the presence of 6 g/L of Phytagel, indicating good synchronisation of the growth of both plant organs.
The somatic embryos of the two spruce species clearly differed in terms of their development. The P. omorika embryos were characterised by more synchronised hypocotyl and radicle growth than that of P. abies embryos. However, the lengths of the hypocotyls and radicle of these germinating embryos generally did not exceed 10 mm and 5 mm, respectively, in contrast to the those of the germinating P. abies somatic embryos (Tables 5, 6), in which the growth of these plant organs was up to 3 times more extensive.
Discussion
The development of somatic embryos of various coniferous tree species is affected mainly by the concentrations of ABA, sucrose or Phytagel (osmoticum) in the maturation medium (Klimaszewska et al. 2000; Iraqi and Tremblay 2001; Teixteira da Silva and Malabadi 2012; Nolan et al. 2014). The concentrations of these components may also be crucial for the further development of the embryos into plants.
Our research showed that the concentration of ABA or sucrose had a significant effect on the production and maturation of P. omorika somatic embryos. In P. abies somatic embryos, this effect was less substantial. The difference in the response of the embryos to the ABA concentration may arise from the greater sensitivity of P. omorika embryos to the presence of this plant hormone in the maturation medium or from different levels of endogenous ABA in the somatic embryos of the two spruce species. The highest number of P. omorika somatic embryos was obtained when the lowest concentration of ABA (10 µM) or sucrose (17 g/L) were applied to the maturation medium. Furthermore, the application of ABA or sucrose at these concentrations contributed to the precocious germination of both P. omorika and P. abies somatic embryos. Most of these embryos had developed hypocotyls, cotyledons and radicles as soon as 3 weeks of cultivation in the presence of 10 µM ABA or 17 g/L sucrose (data not shown). Moreover, chloroplasts were observed in the hypocotyls and cotyledons of these embryos. In contrast, higher concentrations of these components prevented the precocious germination of the somatic embryos of both spruce species (data not shown). According to the data in the literature, ABA was added to the medium at concentrations ranging from 1 to 80 µM to obtain properly developed somatic embryos of conifers (Gjuleva and von Arnold 1999; Garin et al. 2000; Klimaszewska et al. 2000; Iraqi and Tremblay 2001). Harry and Thorpe (1991) applied 40 µM ABA to avoid the precocious germination of Picea rubens somatic embryos. In contrast, Picea glauca and P. mariana somatic embryos did not exhibit a tendency towards precocious germination when 12 µM ABA was applied to the maturation medium (Attree et al. 1990). The sensitivity to ABA differs within the Picea genus and differs even more strongly for more distant species. For example, applying ABA at low concentrations (0–1 µM) stimulated the elongation of Copiapoa tenuissima Ritt. forma mostruosa embryos during the globular stage, whereas applying ABA at high concentrations (10–100 µM) inhibited their growth (Lema-Rumińska et al. 2013).
Similar to the case for ABA, the concentration of sucrose affected the proper development of the somatic embryos. Applying the highest concentration of sucrose (68 g/L), decreased the rates of production and maturation of somatic embryos, particularly P. omorika embryos compared with the results obtained using the lowest sucrose concentration (17 g/L). Despite the similar maturation frequency of embryos of both spruce species, the precocious germination of somatic embryos did not occur upon applying sucrose at 68 g/L. The optimal sucrose concentration in the maturation medium of Picea sp. was reported to be 30 g/L (von Arnold and Hakman 1988; Hakman and von Arnold 1988; Attree et al. 1991). Our results confirmed this finding because optimal development and production of P. abies and P. omorika somatic embryos occurred upon applying 34 g/L of sucrose. Iraqi and Tremblay (2001) demonstrated that 6 % sucrose positively affected the maturation of Picea mariana and P. glauca somatic embryos by increasing the levels of soluble and insoluble proteins and contributing to the development of more epicotyls. The effect of the sucrose concentration on the maturation of somatic embryos depended mainly on the genotype of the cell line (Garin et al. 2000). For example, applying higher concentrations of sucrose (263 and 350 mM) increased the number of Pinus strobus somatic embryos of one line, whereas the number of embryos of the second line was reduced.
In vitro developmental cell fate is governed by factors such as the genetic composition, stress and plant-growth regulators (Lema-Rumińska et al. 2013). Hoth et al. (2002) identified 1354 Arabidopsis thaliana genes that were either up- or down-regulated following ABA treatment (Hoth et al. 2002). Subsequently, Akihiro et al. (2006) identified 27 genes in rice cultures, including starch biosynthesis-related genes, for which expression was induced by combined sucrose and ABA treatment, highlighting the importance of establishing the sucrose and ABA concentrations required for optimal embryonic development, maturation and germination. Moreover, the sugar/osmoticum levels modulate the abscisic acid-independent expression of stress-responsive genes (Déjardin et al. 1999). Chromatin is assumed to integrate stress, hormonal, and developmental pathways, leading to the activation of the embryogenic programme (Fehér 2014). Light-mediated transcriptomic changes might explain the inhibition of hypocotyl growth and the subsequent deficiency of radicle and hypocotyl growth synchronisation because the expression of the chromatin remodelling factor ENHANCED PHOTOMORPHOGENIC1, previously known as PICKLE, which is a repressor of photomorphogenesis in A. thaliana, was specifically repressed in the hypocotyls by light exposure (Jing et al. 2013).
The Phytagel concentration in the maturation medium affected the production of somatic embryos of Picea abies and the maturation of both of the tested spruce species. The highest maturation frequency was obtained upon applying 6 g/L of Phytagel. Some reports suggested that increasing the concentration of a gelling agent from 4 to 8 g/L (Teyssier et al. 2011) or 9 g/L (Morel et al. 2014) promoted the maturation of the somatic embryos of some conifers. Garin et al. (2000) demonstrated that the Phytagel concentration was critical for the maturation of somatic embryos of five tested embryogenic lines of Pinus strobus. The authors obtained a higher efficiency of embryonic maturation when Phytagel was applied at 1.0 % compared with 0.6 %. A similar effect was observed by Klimaszewska et al. (2007) for various pine species when using Phytagel at 12 g/L and by Lelu-Walter and Pâques (2009) for a hybrid larch when using Phytagel at 8 g/L. Increasing the concentration of Phytagel in the maturation media allowed these authors to obtain well-developed somatic embryos capable of germination. High concentrations of gellan gum in the maturation medium were also required for somatic embryos of Pinus pinaster to reach the cotyledonary developmental stage (Morel et al. 2014). Such a phenomenon was not observed in the two Picea species that we tested. The Phytagel concentration might modulate the endogenous ABA content in maturing somatic embryos. Pine embryos maturing in the presence of 9 g/L Phytagel contained more endogenous ABA compared with that of embryos incubated in the presence of 4 g/L Phytagel (Morel et al. 2014). Therefore, a Phytagel-induced increase in the endogenous ABA level may explain why the hypocotyls of P. omorika embryos were the longest specifically when the lowest concentration of Phytagel was used.
Analysis of the starch content of the somatic embryos of P. abies and P. omorika showed that the ABA concentration significantly affected their starch-accumulation pattern during various stages of embryonic development. Notable variations in the starch content were observed particularly in 5-week-old P. omorika somatic embryos that matured in the presence of high concentrations (40 or 80 µM) of ABA. In these embryos, the starch content was more than 3-fold higher than that of embryos treated with the lower concentrations of ABA (10 and 20 µM). However, 2 weeks later, embryos treated with the lower or higher concentration of ABA had similar starch contents. Most likely, during this period, the accumulated starch was rapidly hydrolysed for the further development of the embryos. The effect of the ABA concentrations on the starch contents was more pronounced in P. omorika embryos, similar to the relation between the ABA concentration and the formation and maturation of embryos. These results suggested that P. omorika somatic embryos were definitely more sensitive to the high concentration of ABA than were P. abies embryos. ABA did not affect the starch accumulation of the somatic embryos of Larix x leptoeuropaea (von Aderkas et al. 2002), whereas the starch accumulation of somatic embryos of Camellia sinensis increased according to the concentration of ABA applied (Sharma et al. 2004).
The higher concentrations of sucrose in the medium supported starch storage by both P. abies and P. omorika developing somatic embryos; the higher the concentration of sucrose used, the higher the level of starch in the somatic embryos, irrespective of their developmental stage. Iraqi et al. (2005) also observed a higher rate of starch accumulation in tissues of Picea mariana maturing on 3 and 6 % sucrose-containing media than in tissues matured on a medium containing 1 % sucrose. Additionally, Businge et al. (2013) found that Picea abies somatic embryos treated with 3 % sucrose contained a high level of sucrose and other water stress-related compounds, including raffinose and late embryogenesis abundant protein. Increased starch accumulation was reported when greater sucrose concentrations were added to the maturation medium. The highest starch content was observed when P. abies were grown on medium containing 68 g/L of sucrose. Businge et al. (2013) observed that the starch content of P. abies somatic embryos cultivated on 3 % sucrose ranged from 160 to 220 mg/g DW, depending on the cell line. In our experiments, the starch content of P. abies somatic embryos cultivated with 34 g/L of sucrose reached a maximum of 40 mg/g FW (data not shown). In contrast, their starch content was 120–125 mg/g FW when the osmoticum was 4–8 g/L of Phytagel (data not shown).
P. abies somatic embryos accumulated much more starch than did P. omorika embryos during maturation at the same concentration of sucrose. In our opinion, this result may be due to the lower demand of P. omorika somatic embryos for this type of storage material compared with that of P. abies somatic embryos. Despite this consideration, our results suggested that the concentration of sucrose in the medium affected the dynamics of the accumulation and hydrolysis of starch during the development of somatic embryos of both spruce species. For example, Picea abies somatic embryos maturing on medium supplemented with sucrose at 17 or 34 g/L accumulated starch continuously from 3 to 7 weeks of culture, whereas embryos maturing on medium supplemented with 68 g/L of sucrose began to hydrolyse starch after 5 weeks of culture. These results are in perfect agreement with the findings of Lipavská et al. (2000) that in P. abies somatic embryos, the starch content increased during approximately 5–6 weeks of cultivation on maturation medium and then, the starch content clearly decreased during weeks 7–8 of cultivation. Compared with this pattern, the pattern of accumulation and mobilisation of starch in P. abies somatic embryos that matured in the presence of 68 g/L of sucrose appeared to be more appropriate for embryonic development. This hypothesis was confirmed by our observation that during the germination of P. abies somatic embryos, the embryos derived from the cultures supplemented with 68 g/L of sucrose produced better developed roots and were characterised by the improved synchronisation of the hypocotyl and root growth compared with those of embryos that matured at a lower concentration of sucrose.
Our study showed that manipulating the osmoticum in the medium through applying various concentrations of Phytagel also affected starch accumulation in the cells of maturing somatic embryos of both spruce species. For example, P. omorika somatic embryos contained much more starch when matured on medium containing Phytagel at the lower concentrations (4 or 6 g/L). At the highest concentration of Phytagel (8 g/L), the starch-accumulation process was inhibited. This result suggested that too high a level of an osmoticum in the medium limited the transport of sucrose from the medium to the somatic embryo, and therefore, the level of starch reserves in the developing embryos was reduced.
The P. abies somatic embryos accumulated significantly more starch than did the P. omorika embryos matured in the presence of the same concentrations of Phytagel. This finding is the same as was observed in the case of embryos matured in the presence of various concentrations of sucrose. Recent research has shown that the concentration of Phytagel significantly affected the maturation of the somatic embryos of some conifer species (Garin et al. 2000; Klimaszewska et al. 2007; Lelu-Walter and Pâques 2009; Teyssier et al. 2011). However, the published literature did not address whether this component of the maturation medium affected starch accumulation in the somatic embryos. Our results provide additional knowledge about the effect of the Phytagel concentration on the physiology of maturation of conifer somatic embryos.
Some authors reported that ABA or osmotic-stress treatment during the maturation of conifer somatic embryos affected their ability for further development (Hogberg et al. 2001). In our study, we did not observe a stimulatory or inhibitory effect of ABA on the germination of P. abies somatic embryos (the experiment was not conducted using P. omorika somatic embryos). However, in the case of osmotic-stress treatment, we noticed that the concentration of both sucrose and Phytagel in the maturation medium had an effect, in some cases, on the germination of Picea abies and P. omorika somatic embryos. Although in our experiments, the presence of sucrose at the highest concentration in the medium reduced the abilities of the tested line for the production and maturation of somatic embryos, during germination, sucrose at the highest concentration improved the radicle growth of embryos of both spruce species. We observed that the growth of hypocotyls and radicles of germinating P. omorika embryos was dependent on the Phytagel dose in the maturation medium.
Our findings are partially consistent with the results obtained by other authors (Iraqi and Tremblay 2001; Lelu-Walter and Pâques 2009). Additionally, our study showed that the effect of the osmoticum on the germination of spruce somatic embryos was also dependent on the tree species and the genotype of the embryogenic-tissue line.
In general, our studies demonstrated that the response of spruce embryogenic-tissue lines to the concentration of the three tested components in the maturation medium was highly dependent on the species. The concentration of both ABA and the osmoticum in the medium affected the pattern of starch accumulation in the developing embryos of the tested spruce species. These factors may be the keys to the further development of plant embryos. Based on our findings, the quality of the Picea sp. somatic embryos was enhanced by adding 68 g/L of sucrose or 6 g/L of Phytagel to the maturation medium. Adding sucrose or Phytagel to the cultivation medium at these concentrations improved the starch-accumulation process and radicle growth, whereas the ABA dose during the maturation stage was less important. The presented results provide new information on the effects of ABA and osmoticum on the starch accumulation pattern of Picea abies and P. omorika somatic embryos.
Author contribution statement
THP conceived and designed research. THP conducted experiments. EMK and ER performed starch content analysis. THP and EMK analysed data. THP, EMK and KB wrote the manuscript. All authors scrutinized and corrected the manuscript.
Abbreviations
- ABA:
-
Abscisic acid
- IBA:
-
Indolile-3-butyric acid
- KOH:
-
Potassium hydroxide
- LED:
-
Light-emitting diode
- LM:
-
Litvay medium
- ME:
-
Margara medium
- Picloram:
-
4-Amino-3,5,6-trichloropicolinic acid
References
Akihiro T, Umezawa T, Ueki C, Lobna BM, Mizuno K, Ohta M, Fujimura T (2006) Genome wide cDNA-AFLP analysis of genes rapidly induced by combined sucrose and ABA treatment in rice cultured cells. FEBS Lett 580:5947–5952
Attree SM, Budimir S, Fowke LC (1990) Somatic embryogenesis and plantlet regeneration from cultured shoots and cotyledons of seedlings from stored seeds of black and white spruce (Picea mariana and P. glauca). Can J Bot 68:30–34
Attree SM, Moore D, Sawhney VK, Fowke LC (1991) Enhanced maturation and desiccation tolerance of white spruce (Picea glauca (Moench.) Voss) somatic embryos: effects of non-plasmolysing water stress and abscisic acid. Ann Bot 68:519–525
Ballian D, Longauer R, Mikić T, Paule L, Kajba D, Gömöry D (2006) Genetic structure of rare European conifer, Serbian spruce (Picea omorika (Panč.) Purk.). Plant Syst Evol 260:53–63
Bonga JM, Klimaszewska K, von Aderkas P (2010) Recalcitrance in clonal propagation, in particular of conifers. Plant Cell Tiss Organ Cult 100:241–254
Bříza J, Pavingerová D, Vlasák J, Niedermeierová H (2013) Norway spruce (Picea abies) genetic transformation with modified Cry 3A gene of Bacillus thuringiensis. Acta Biochim Pol 60:395–400
Budimir S, Vujičić R (1992) Benzyladenine induction of buds and somatic embryogenesis in Picea omorika (Pancić) Purk. Plant Cell Tiss Org Cult 31:89–94
Businge E, Bygdell J, Wingsle G, Moritz T, Egertsdotter U (2013) The effects of carbohydrates and osmoticum on storage reserve accumulation and germination of Norway spruce somatic embryos. Physiol Plant 149:273–285
Chalupa V (1985) Somatic embryogenesis and plantlet regeneration from cultured immature and mature embryos of Picea abies (L.) Karst Comm Inst. Forest 14:57–63
Déjardin A, Sokolov LN, Kleczkowski LA (1999) Sugar/osmoticum levels modulate differential abscisic acid-independent expression of two stress-responsive sucrose synthase genes in Arabidopsis. Biochem J 2:503–509
Deo PC, Tyagi AP, Taylor M, Harding R, Becker D (2010) Factors affecting somatic embryogenesis and transformation in modern plant breeding. South Pac J Nat Appl Sci 28:27–40
Fehér A (2014) Somatic embryogenesis—stress-induced remodeling of plant cell fate. Biochim Biophys Acta 4:385–402
Garin E, Bernier-Cardou M, Isabel N, Klimaszewska K, Plourde A (2000) Effect of sugars, amino acids, and culture technique on maturation of somatice embryos of Pinus strobus on medium with two gellan gum concentrations. Plant Cell Tiss Org Cult 62:27–37
Gjuleva V, von Arnold S (1999) Maturation capacity of somatic embryos of Picea abies after prolonged proliferation culture. Biol Plant 2:161–168
Gutmann M, von Aderkas P, Label P, Lelu MA (1996) Effect of abscisic acid on somatic embryo maturation of hybrid larch. J Exp Bot 47:1905–1917
Hakman I, von Arnold S (1988) Somatic embryogenesis and plant regeneration from suspension cultures of Picea glauca (white spruce). Physiol Plant 72:579–587
Hakman I, Fowke LC, von Arnold S, Eriksson T (1985) The development of somatic embryos in tissue cultures initiated from immature embryos of Picea abies (Norway spruce). Plant Sci 38:53–59
Harry IS, Thorpe TA (1991) Somatic embryogenesis and plantlet regeneration from mature zygotic embryos of red spruce. Bot Gaz 152:446–452
Hazubska T, Szczygieł K (2003) Induction of somatic embryogenesis in spruce: Picea omorika, P. pungens ‘Glauca’, P. breweriana and P. abies. Dendrobiology 50:17–24
Hazubska-Przybył T, Bojarczuk K (2008) Somatic embryogenesis of selected spruce species (Picea abies, P. omorika, P. pungens ‘Glauca’ and P. breweriana). Acta Soc Bot Pol 77:189–199
Hazubska-Przybył T, Bojarczuk K, Guzicka M (2008) Structure of embryogenic tissues and accumulation of storage materials in somatic embryos of Picea abies and P. omorika. Dendrobiology 60:19–28
Hazubska-Przybył T, Chmielarz P, Michalak M, Bojarczuk K (2010) Cryopreservation of embryogenic tissues of Picea omorika (Serbian spruce). Plant Cell Tiss Org Cult 102:35–44
Hazubska-Przybył T, Chmielarz P, Michalak M, Dering M, Bojarczuk K (2013) Survival and genetic stability of Picea abies embryogenic cultures after cryopreservation using a pregrowth-dehydration method. Plant Cell Tiss Organ Cult 113:303–313
Hogberg KA, Bozhkov PV, Gronroos R, von Arnold S (2001) Critical factors affecting ex vitro performance of somatic embryo plants of Picea abies. Scand J For Res 16:295–304
Hoth S, Morgante M, Sanchez JP, Hanafey MK, Tingey SV, Chua NH (2002) Genome-wide gene expression profiling in Arabidopsis thaliana reveals new targets of abscisic acid and largely impaired gene regulation in the abi1-1 mutant. J Cell Sci 115:4891–4900
Huber S, Israel D (1982) Biochemical basis for portioning of photosynthetically fixed carbon between starch and sucrose in soybean (Glycine max Merr.) leaves. Plant Physiol 69:691–696
Iraqi D, Tremblay FM (2001) The role of sucrose during maturation of black spruce (Picea mariana) and white spruce (Picea glauca) somatic embryos. Physiol Plant 111:381–388
Iraqi D, Le VQ, Lamhamedi MS, Tremblay FM (2005) Sucrose utilization during embryo development in black spruce: involvement of apoplastic invertase in the tissue and of extracellular invertase in the medium. J Physiol 162:115–124
Jing Y, Zhang D, Wang X, Tang W, Wang W, Huai J, Xu G, Chen D, Li Y, Lin R (2013) Arabidopsis chromatin remodeling factor PICKLE interacts with transcription factor HY5 to regulate hypocotyl cell elongation. Plant Cell 25:242–256
Kępczyńska E (2006) In vitro germination and conversion of somatic embryos. Biotechnologia 4:78–94
Kermode AR (2005) Role of abscisic acid in seed dormancy. J Plant Growth Regul 24:319–344
Kim YW, Youn J, Noh ER, Kim JC (1999) Somatic embryogenesis and plant regeneration from immature zygotic embryos of Japanese larch (Larix leptolepis). Plant Cell Tiss Org Cult 55:95–101
Klimaszewska K, Cardou MB, Cyr DR, Sutton BCS (2000) Influence of gelling agents on culture medium gel strength, water availability, tissue water potential, and maturation response in embryogenic cultures of Pinus strobus L. In Vitro Cell Dev Biol-Plant 36:279–286
Klimaszewska K, Trontin JF, Becwar M, Devillard C, Park YS, Lelu-Walter MA (2007) Recent progress in somatic embryogenesis of four Pinus spp. Tree For Sci Biotech 1:11–25
Kong L, von Aderkas P (2007) Genotype effect on ABA consumption and somatic embryo maturation in interior spruce (Picea glauca x engelmanni). J Exp Bot 58:1525–1531
Leljak-Levanić D, Mihaljević S, Jelaska S (2009) Variation in DNA methylation in Picea omorika (Panč) Purk. Embryogenic tissue and the ability for embryo maturation. Propag Ornam Plants 9:3–9
Lelu-Walter MA, Pâques LE (2009) Simplified and improved somatic embryogenesis of hybrid larches (Larix x eurolepis and Larix x marschlinsii). Perspectives for breeding. Ann For Sci 66:104
Lema-Rumińska J, Goncerzewicz K, Gabriel M (2013) Influence of abscisic acid and sucrose on somatic embryogenesis in cactus Copiapoa tenuissima Ritt. forma monstruosa. The Scientific World Journal vol. 2013, p 7. Hindawi Publishing Corporation (Article ID 513985)
Lipavská H, Svobodová H, Albrechtová J, Kumstýřová L, Vágner M, Vondráková Z (2000) Carbohydrate status during somatic embryo maturation in Norway spruce. In Vitro Cell Dev Biol-Plant 36:260–267
Litvay JD, Verma DC, Johson MA (1985) Influence of loblolly pine (Pinus taeda L.) culture medium and its components on growth and somatic embryogenesis of wild carrot (Daucus carota L.). Plant Cell Rep 4:325–328
Malá J, Cvikrová M, Máchová P, Martincová O (2009) Polyamines during somatic embryo development in Norway spruce (Picea abies [L.]). J Forest Sci 55:75–80
Margara J (1977) La multiplication vegetative de la betterave (Beta vulgaris L.) en culture in vitro. CR Acad Sc Paris 285:1041–1044
Misra S (1994) Conifer zygotic embryogenesis, somatic embryogenesis and seed germination: biochemical and molecular advances. Seed Sci Res 4:357–384
Morel A, Teyssier C, Trontin JF, Eliášová K, Pešek B, Beaufour M, Morabito D, Boizot N, Le Metté C, Belal-Bessai L, Reymond I, Harvengt L, Cadene M, Corbineau F, Vágner M, Label P, Lelu-Walter MA (2014) Early molecular events involved in Pinus pinaster Ait. somatic embryo development under reduced water availability: transcriptomic and proteomic analyses. Physiol Plant 152:184–201
Nolan KE, Song Y, Liao S, Saeed NA, Zhang X, Rose RJ (2014) An unusual abscisic acid and gibberellic acid synergism increases somatic embryogenesis, facilitates its genetic analysis and improves transformation in Medicago truncatula. PLoS One 9(6):e99908
Rai MK, Shehawat NS, Harish Gupta AK, Phulwaria M, Ram K, Jaiswal U (2011) The role of abscisic acid in plant tissue culture: a review of recent progress. Plant Cell Tiss Organ Cult 106:179–190
Salopek B, Tramiśak-Milaković T, Mihaljević S, Jelaska S (1997) Storage product accumulation during the maturation of Picea omorika (Panč.) Purk. somatic embryos. Period Biol 99:117–124
Sauer N (2007) Molecular physiology of higher plant sucrose transporters. FEBS Lett 581:2309–2317
Sharma P, Pandey S, Bhattacharya A, Nagar PK, Ahuja PS (2004) ABA associated biochemical changes during somatic embryo development in Camellia sinensis (L.) O. Kuntze. J Plant Physiol 161:1269–1276
Somogyi M (1952) Notes on sugar determination. J Biol Chem 195:19–23
Sun H, Aidun CK, Egertsdotter U (2011) Possible effect from shear stress on maturation of somatic embryos of Norway spruce (Picea abies). Biotechnol Bioeng 108:1089–1099
Szczygieł K, Hazubska-Przybył T, Bojarczuk K (2007) Somatic embryogenesis of selected coniferous tree species of the genera Picea, Abies and Larix. Acta Soc Bot Pol 7:7–15
Teixeira da Silva JA, Malabadi RB (2012) Factors affecting somatic embryogenesis in conifers. J For Res 23:503–515
Teyssier C, Grondin C, Bonhomme L, Lomenech AM, Vallance M, Morabito D, Label P, Lelu-Walter AM (2011) Increased gelling agent concentration promotes somatic embryo maturation in hybrid larch (Larix × eurolepsis): a 2-DE proteomic analysis. Physiol Plant 141:156–165
Tramisak-Milaković T, Mihaljević S, Jelaska S (1999) Effects of abscisic acid and carbohydrates on the maturation of Picea omorika (Panč.) Purk. somatic embryos. Acta Bot Croat 58:87–97
von Aderkas P, Rohr R, Sundberg B, Gutmann M, Dumont-Béboux N, Lelu AM (2002) ABA and its influence on development of the embryonal radicle cap, storage product and secondary metabolite accumulation in hybrid larch somatic embryos. Plant Cell Tiss Organ Cult 69:111–120
von Arnold S, Hakman I (1988) Regulation of somatic embryo development in Picea abies by abscisic acid (ABA). J Plant Physiol 132:164–169
Vujičić R, Budimir S (1995) Somatic embryogenesis and plant regeneration in Picea omorika. In: Jain S, Gupta P, Newton R (eds) Somatic embryogenesis in woody plants, vol 3. Kluwer Academic Publishers, Netherlands, pp 81–97
Wind J, Smeekens S, Hanson J (2010) Sucrose: metabolite and signaling molecule. Phytochemistry 71:1610–1614
Acknowledgments
This study was supported by the National Science Centre of Krakow, Poland (Narodowe Centrum Nauki, NCN, Grant Number N N309 130837).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Communicated by J. van Staden.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Hazubska-Przybył, T., Kalemba, E.M., Ratajczak, E. et al. Effects of abscisic acid and an osmoticum on the maturation, starch accumulation and germination of Picea spp. somatic embryos. Acta Physiol Plant 38, 59 (2016). https://doi.org/10.1007/s11738-016-2078-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11738-016-2078-x