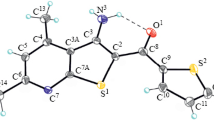
The condensation of 3-[(alkylsulfanyl)methyl]pentane-2,4-diones with aniline in the presence of catalytic amounts of acetic acid led to the formation of 1,1',1''-(6-methyl-1-phenyl-1,2,3,4-tetrahydropyridine-3,3,5-triyl)triethanone. The reaction is presumably a tandem process, incorporating the formation of β-enaminone, elimination of the alkanethiol molecule from the β-enaminone and the starting pentane-2,4-dione, and a subsequent [4+2] cycloaddition of the resulting intermediates. The mechanism of the conversion may also involve intramolecular cyclization of the Michael addition products of 3-[(alkylsulfanyl)methyl]pentane-2,4-dione to 3-(imidoyl)but-3-en-2-one, formed from β-enaminones during the elimination of alkanethiols.

Similar content being viewed by others
References
(a) Kesting, J. R.; Tolderlund, I.; Pedersen, A. F.; Witt, M.; Jaroszewski, J. W.; Staerk, D. J. Nat. Prod. 2009, 72, 312. (b) O'Hagan, D. Nat. Prod. Rep. 2000, 17, 435. (c) Khan, Md. M.; Khan, S.; Saigal; Iqbal, S. RSC Adv. 2016, 6, 42045. (d) Dudognon, Y.; Rodriguez, J.; Constantieux, T.; Bugaut, X. Eur. J. Org. Chem. 2018, 2432. (e) Singh, S.; Gupta, A.; Kapoor, K. K. Synth. Commun. 2020, 50, 1056.
(a) Mohsin, N.; Ahmad, M. Turk. J. Chem. 2018, 42, 1191. (b) Khan, B. A.; Ashfaq, M.; Muhammad, S.; Munawar, K. S.; Tahir, M. N.; Al-Sehemi, A. G.; Alarfaji, S. S. ACS Omega 2022, 7, 29452. (c) Montes-Avila, J.; Delgado-Vargas, F.; Díaz-Camacho, S. P.; Rivero, I. A. RSC Adv. 2012, 2, 1827. (d) Gedeon, S.; Montgomery, A.; Gangapuram, M.; Redda, K. K.; Ardley, T. W. Clin. Med. Rev. Reports 2023, 4, 0135. URL: https://www.auctoresonline.org/article/the-chemistry-andpharmacology-of-tetrahydropyridines-part-2 (e) McAteer, C. H.; Balasubramanian, M.; Murugan, R. In Comprehensive Heterocyclic Chemistry III; Katritzky, A. R.; Ramsden, C. A.; Scriven, E. F. V.; Taylor, R. J. K., Eds.; Elsevier: New York, 2008, Vol. 7, p. 309. (f) Prachayasittikul, S.; Worachartcheewan, A.; Lawung, R.; Ruchirawat, S.; Prachayasittikul, V. EXCLI Journal 2009, 8, 107.
(a) Saini, A.; Kumar, S.; Sandhu, J. S. J. Sci. Ind. Res. 2008, 67, 95. (b) Prajapati, N. P.; Vekariya, R. H.; Patel, H. D. Synth. Commun. 2015, 45, 2399. (c) Sharma, M. G.; Rajani, D. P.; Patel, H. M. R. Soc. Open Sci. 2017, 4, 170006.
(a) Yelwande, A. A.; Dinore, J. M.; Mule, P. A.; Dobhal, B. S. J. Biol. Chem. Chron. 2019, 5(3), 163. (b) Petrović, Z. D.; Simijonović, D.; Đorović, J.; Milovanović, V.; Marković, Z.; Petrović, V. P. ChemistrySelect 2017, 2, 11187. (c) Bharti, R.; Parvin, T. J. Heterocycl. Chem. 2015, 52, 1806. (d) Ramin, G.-R.; Hajar S. C. R. Chimie 2013, 16, 1047. (e) Mishra, S.; Ghosh, R. Tetrahedron Lett. 2011, 52, 2857. (f) Clarke, P. A.; Zaytzev, A. V.; Whitwood, A. C. Tetrahedron Lett. 2007, 48, 5209. (g) Khan, A. T.; Parvin, T.; Choudhury, L. H. J. Org. Chem. 2008, 73, 8398. (h) Wang, H.-J.; Mo, L.-P.; Zhang, Z.-H. ACS Comb. Sci. 2011, 13, 181. (i) Kireeva, D. R.; Kamalova, A. I. Russ. J. Org. Chem. 2020, 56, 1733. (j) Noël, R.; Fargeau-Bellassoued, M.-C.; Vanucci-Bacqué, C.; Lhommet, G. Synfacts. 2008, 9, 920. (k) Keller, P. A. In Comprehensive Heterocyclic Chemistry III; Katritzky, A. R.; Ramsden, C. A.; Scriven, E. F. V.; Taylor, R. J. K., Eds.; Elsevier: New York, 2008, Vol. 7, p. 217.
(a) Baeva, L. A.; Nugumanov, R. M.; Fatykhov, A. A.; Lyapina, N. K. Russ. J. Org. Chem. 2018, 54, 444. (b) Baeva, L. A.; Gataullin, R. R. Russ. J. Org. Chem. 2021, 57, 1167.
Duan, Z.; Zhao, W.; Yang, F. CN Patent 101792412.
(a) Yamauchi, M.; Katayama, S.; Watanabe, T. Synthesis 1982, 935. (b) Gao, M.; Willis, M. C. Org. Lett. 2017, 19, 2734. (c) Horhant, D.; Le Lamer, A.; Boustie, J.; Uriac, P.; Gouault, N. Tetrahedron Lett. 2007, 48, 6031. (d) Vostrikov, N. S.; Spirikhin, L. V.; Lobov, A. N.; Gimazetdinov, A. M.; Zileeva, Z. R.; Vakhitova, Y. V.; Macaev, Z. R.; Pivnitsky, K. K.; Miftakhov, M. S. Mendeleev Commun. 2019, 29, 372. (e) Tarsis, E.; Gromova, A.; Lim, D.; Zhou, G.; Coltart, D. M. Org. Lett. 2008, 10, 4819. (f) Barrett, A. G. M.; Kamimura, A. J. Chem. Soc., Chem. Commun. 1995, 1755.
Eshghi, H.; Seyedi, S. M.; Safaei, E.; Vakili, V.; Farhadipour, A.; Bayat-Mokhtari, M. J. Mol. Catal. A: Chem. 2012, 363-364, 430.
Pretsch, E.; Buhlmann, P.; Badertscher, M. Structure Determination of Organic Compounds. Tables of Spectral Data; Springer-Verlag: Berlin, Heidelberg, 2009.
(a) Baeva, L. A.; Biktasheva, L. F.; Fatykhov, A. A.; Lyapina, N. K. Russ. J. Org. Chem. 2013, 49, 1283. (b) Baeva, L. A.; Biktasheva, L. F.; Gataullin R. R.; Nugumanov, T. R. Russ. J. Org. Chem. 2023, 59, 1008.
Author information
Authors and Affiliations
Corresponding author
Additional information
Translated from Khimiya Geterotsiklicheskikh Soedinenii, 2024, 60(1/2), 107–110
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Baeva, L.A., Gataullin, R.R. & Nugumanov, R.M. A synthesis of triacetyl-substituted 1,2,3,4-tetrahydropyridine by the reaction of 3-[(alkylsulfanyl)methyl]pentane-2,4-diones with aniline. Chem Heterocycl Comp 60, 107–110 (2024). https://doi.org/10.1007/s10593-024-03301-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10593-024-03301-7