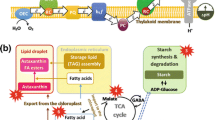
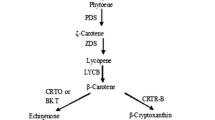
Abstract
Under stressful environments, many green algae such as Haematococcus pluvialis accumulate secondary ketocarotenoids such as canthaxanthin and astaxanthin. The carotenogenesis, responsible for natural phenomena such as red snows, generally accompanies larger metabolic changes as well as morphological modifications, i.e., the conversion of the green flagellated macrozoids into large red cysts. Astaxanthin accumulation constitutes a convenient way to store energy and carbon, which will be used for further synthesis under less stressful conditions. Besides this, the presence of high amount of astaxanthin enhances the cell resistance to oxidative stress generated by unfavorable environmental conditions including excess light, UV-B irradiation, and nutrition stress and, therefore, confers a higher survival capacity to the cells. This better resistance results from the quenching of oxygen atoms for the synthesis itself as well as from the antioxidant properties of the astaxanthin molecules. Therefore, astaxanthin synthesis corresponds to a multifunctional response to stress. In this contribution, the various biochemical, genetic, and molecular data related to the biosynthesis of ketocarotenoids by Haematococcus pluvialis and other taxa are reviewed and compared. A tentative regulatory model of the biochemical network driving astaxanthin production is proposed.






Similar content being viewed by others
Abbreviations
- BKT, CRTO:
-
4,4′-Ketolase
- Car:
-
Carotenoid
- Chl:
-
Chlorophyll
- CHX:
-
Cycloheximide
- CHY-b, CRTR-b, CRTZ:
-
3,3′-Hydroxylase
- CPTA:
-
2-(4-Chlorophenylthio)-triethylamine
- CRTL-b:
-
Lycopene β-cyclase
- DMAPP:
-
Dimethylallyl pyrophosphate
- DPA:
-
Diphenylamine
- GGPP:
-
Geranylgeranyl pyrophosphate
- HL:
-
High-light
- IPP:
-
Isopentenylpyrophosphate
- IPI:
-
Isopentenylpyrophosphate isomerase
- LHC:
-
Light-harvesting complexes
- MGDG:
-
Monogalactosyldiacylglycerol
- NF:
-
Norflurazon
- PAGE:
-
Polyacrylamide gel electrophoresis
- PDS:
-
Phytoene desaturase
- PQ:
-
Plastoquinone
- PS:
-
Photosystem
- PSY:
-
Phytoene synthase
- PTOX:
-
Plastid terminal oxidase of chlororespiration
- ROS:
-
Reactive oxygen species
- SC:
-
Secondary carotenoid
- SOD:
-
Superoxide dismutase
- TAG:
-
Triacylglycerol
- ZDS:
-
ζ-Carotene desaturase
References
Abe K, Takizawa H, Kimura S, Hirano M (2004) Characteristics of chlorophyll formation of the aerial microalga Coelastrella striolata var. multistriata and its application for environmental biomonitoring. J Biosci Bioeng 98:34–39
Abe K, Hattori H, Hirano M (2007) Accumulation and antioxidant activity of secondary carotenoids in the aerial microalga Coelastrella striolata var. multistriata. Food Chem 100:656–661
Allen JF (1993) Control of gene expression by redox potential and the requirement for chloroplast and mitochondrial genomes. J Theor Biol 165:609–631
Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Biol 55:373–399
Apostol I, Heinstein PF, Low PS (1989) Rapid stimulation of an oxidative burst during elicitation of cultured plant cells. Role in defense and signal transduction. Plant Physiol 90:109–116
Asada K (1994) Production and action of active oxygen species in photosynthetic tissues. In: Foyer CH, Mullineaux PM (eds) Causes of photooxidative stress and amelioration of defense systems in plants. CRC, Boca Raton, pp 77–104
Asada K (1999) The water-water cycle in chloroplasts: scavenging of active oxygens and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol 50:601–639
Bar E, Rise M, Vishkautsan M, Arad SM (1995) Pigments and structural changes in Chlorella zofingiensis upon light and nitrogen stress. J Plant Physiol 146:527–534
Barr J, White WS, Chen L, Bae S, Rodermel S (2004) The GHOST terminal oxidase regulates developmental programming in tomato fruit. Plant Cell Environ 27:840–852
Beck CF (2005) Signaling pathways from the chloroplast to the nucleus. Planta 222:743–756
Ben-Amotz A, Avron M (1983) On the factors which determine massive β-carotene accumulation in the halotolerant alga Dunaliella bardawil. Plant Physiol 72:593–597
Ben-Amotz A, Shaish A, Avron M (1989) Mode of action of the massively accumulated β-carotene of Dunaliella bardawil in protecting the alga against damage by excess irradiation. Plant Physiol 91:1040–1043
Bidigare RR, Ondrusek ME, Kennicutt MC, Iturriaga R, Harvey HR, Haham RW, Macko SA (1993) Evidence for a photo-protective function for secondary carotenoids of snow algae. J Phycol 29:427–434
Bjerkeng B (1997) Chromatographic analysis of synthesized astaxanthin—a handy tool for the ecologist and the forensic chemist? Prog Fish Cult 59:129–140
Blokhina O, Virolainen E, Fagerstedt KV (2003) Antioxidants, oxidative damage and oxygen deprivation stress: a review. Ann Bot 91:179–194
Boussiba S (2000) Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiol Plant 108:111–117
Boussiba S, Fan L, Vonshak A (1992) Enhancement and determination of astaxanthin accumulation in green alga Haematococcus pluvialis. Methods Enzymol 213:386–391
Boussiba S, Bing W, Yuan JP, Zarka A, Chen F (1999) Changes in pigment profiles of Haematococcus pluvialis during response to environmental stress. Biotechnol Lett 21:601–604
Breitenbach J, Misawa N, Kajiwara S, Sandmann G (1996) Expression in Escherichia coli and properties of the carotene ketolase from Haematococcus pluvialis. FEMS Microbiol Lett 140:241–246
Breitenbach J, Zhu CF, Sandmann G (2001) Bleaching norflurazon inhibits phytoene desaturase by competition with the cofactors. J Agric Food Chem 49:5270–5272
Brinda BR, Sarada R, Kamath BS, Ravishankar GA (2004) Accumulation of astaxanthin in flagellated cells of Haematococcus pluvialis—cultural and regulatory aspects. Curr Sci 87:1290–1295
Britton G, Liaaen-Jensen S, Pfander H (2008) Carotenoids. Handbook. Birkhäuser Verlag, Basel
Brown TE, Richardson FL, Vaughn ML (1967) Development of red pigmentation in Chlorococcum wimmeri (Chlorophyta: Chlorococcales). Phycologia 6:167–184
Burzyk J (1987) Cell wall carotenoids in green algae which form sporopollenins. Phytochem 26:121–128
Carlson M (1999) Glucose repression in yeast. Curr Opin Microbiol 2:202–207
Carol P, Stevenson D, Bisanz C, Breitenbach J, Sandmann G, Mache R, Coupland G, Kuntz M (1999) Mutations in the Arabidopsis gene IMMUTANS cause a variegated phenotype by inactivating a chloroplast terminal oxidase associated with phytoene desaturation. Plant Cell 11:57–68
Ceron MC, García-Malea MC, Rivas J, Acien FG, Fernandez JM, Del Río E, Guerrero MG, Molina E (2007) Antioxidant activity of Haematococcus pluvialis cells grown in continuous culture as a function of their carotenoid and fatty acid content. Appl Microbiol Biotechnol 74:1112–1119
Chamovitz D, Pecker I, Hirschberg J (1993) Molecular and biochemical characterization of herbicide-resistant mutants of cyanobacteria reveals that phytoene desaturation is a rate limiting step in carotenoids biosynthesis. J Biol Chem 268:17348–17353
Chandel NS, Maltepe E, Goldwasser E, Mathieu CE, Simon MC, Schumacker PT (1998) Mitochondrial reactive oxygen species trigger hypoxia-induced transcription. Proc Natl Acad Sci USA 95:11715–11720
Coesel SN, Baumgartner AC, Teles LM, Ramos AA, Henriques NM, Cancela L, Varela JCS (2008) Nutrient limitation is the main regulatory factor for carotenoid accumulation and for psy and pds steady state transcript levels in Dunaliella salina (Chlorophyta) exposed to high light and salt stress. Mar Biotechnol 10:602–611
Cui B, Zhong F (2008) Effects of plant growth regulators on growth and astaxanthin content in green alga Haematoccus pluvialis. Fisheries Sci 27:478–482
Cunningham F, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49:557–583
Cunningham F, Gantt E (2005) A study in scarlet: enzymes of ketocarotenoid biosynthesis in the flowers of Adonis aestivalis. Plant J 41:478–492
Czerpak R, Piotrowska A, Szulecka K (2006) Jasmonic acid affects changes in the growth and some components content in alga Chlorella vulgaris. Acta Physiol Plant 28:195–203
Del Campo JAD, Moreno J, Rodrıguez H, Angeles Vergas M, Rivas J, Guerrero MG (2000) Carotenoid content of chlorophycean microalgae: factors determining lutein accumulation in Muriellopsis sp. (Chlorophyta). J Biotechnol 76:51–59
Del Río E, Acién FG, García-Malea MC, Rivas J, Molina-Grima E, Guerrero MG (2005) Efficient one-step production of astaxanthin by the microalga Haematococcus pluvialis in continuous culture. Biotechnol Bioeng 91:808–815
Diversé-Pierluissi M, Krogmann DW (1988) A zeaxanthin protein from Anacystis nidulans. Biochim Biophys Acta 933:372–377
Dong QL, Zao XM, Xing XY, Hu JZ, Gong JX (2007) Mechanism of salt stress inducing astaxanthin synthesis in Haematococcus pluvialis. Huaxue Gong Cheng 35:45–47
Droop MR (1955a) Carotenogenesis in Haematococcus pluvialis. Nature 175:42–43
Droop MR (1955b) Some factors governing encystment in Haematocccus pluvialis. Arch Mikrobiol 21:267–272
Escoubas J-M, Lomas M, LaRoche J, Falkowski PG (1995) Light intensity regulation of cab gene transcription is signaled by the redox state of the plastoquinone pool. Proc Natl Acad Sci USA 92:10237–10241
Fan L, Vonshak A, Boussiba S (1994) Effect of temperature and irradiance on growth of Haematococcus pluvialis (Chlorophyceae). J Phycol 30:829–833
Fan L, Vonshak A, Zarka A, Boussiba S (1998) Does astaxanthin protect Haematococcus against light damage? Z Naturforsch 53c:93–100
Fassett RG, Coombes JS (2009) Astaxanthin, oxidative stress, inflammation and cardiovascular disease. Future Cardiol 5:333–342
Foyer CH, Mullineaux PM (1994) Causes of photooxidative stress and amelioration of defense systems in plants. CRC Press, Boca Raton
Fraser PD, Bramley PM (2004) The biosynthesis and nutritional uses of carotenoids. Prog Lipid Res 43:228–265
Fraser PD, Miura Y, Misawa N (1997) In vitro characterization of astaxanthin biosynthetic enzymes. J Biol Chem 272:6128–6135
Fraser PD, Shimada H, Misawa N (1998) Enzymic confirmation of reactions involved in routes to astaxanthin formation, elucidated using a direct substrate in vitro assay. Eur J Biochem 252:229–236
Green J (1963) The occurrence of astaxanthin in the euglenoid Trachelomonas volvocina. Comp Biochem Physiol 9:313–316
Grünewald K, Hagen C (2000) Extrusion of secondary carotenoid containing vesicles from flagellates of Haematococcus pluvialis (Volvocales; Chlorophyceae). J Appl Bot 74:141–144
Grünewald K, Hagen C (2001) β-carotene is the intermediate exported from the chloroplast during accumulation of secondary carotenoid in Haematococcus pluvialis. J Appl Phycol 13:89–93
Grünewald K, Hagen C, Braune K (1997) Secondary carotenoid accumulation in flagellates of the green alga Haematococcus lacustris. Eur J Phycol 32:387–392
Grünewald K, Eckert M, Hirschberg J, Hagen C (2000) Phytoene desaturase is localized exclusively in the chloroplast and up-regulated at the mRNA level during accumulation of secondary carotenoids in Haematococcus pluvialis (Volvocales, Chlorophyceae). Plant Physiol 122:1261–1268
Grünewald K, Hirschberg J, Hagen C (2001) Ketocarotenoid biosynthesis outside of plastids in the unicellular green alga Haematococcus pluvialis. J Biol Chem 276:6023–6029
Grung M, Metzger P, Liaaen-jensen S (1989) Algal carotenoids 42. Secondary carotenoids of algae. 1. Primary and secondary carotenoids in two races of the green alga Botryococcus braunii. Biochem Syst Ecol 17:263–269
Grung M, D’Souza FML, Borowitzka M, Liaaen-Jensen S (1992) Algal carotenoids. 51. Secondary carotenoids. 2. Haematococcus pluvialis aplanospores as a source of (3S, 3′S)-astaxanthin esters. J Appl Phycol 4:165–171
Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biochem Sci 21:210–216
Hagen C, Braune W, Birckner E, Nüske J (1993a) Functional aspects of secondary carotenoids in Haematococcus lacustris (Girod) Rostafinski (Volvocales). I. The accumulation period as an active metabolic process. New Phytol 125:625–633
Hagen C, Braune W, Greulich F (1993b) Functional aspects of secondary carotenoids in Haematococcus lacustris [Girod] Rostafinski (Volvocales). IV. Protection from photodynamic damage. J Photochem Photobiol B 20:153–160
Hagen C, Grünewald K, Schmidt S, Muller J (2000) Accumulation of secondary carotenoids in flagellates of Haematococcus pluvialis (Chlorophyta) is accompanied by an increase in per unit chlorophyll productivity of photosynthesis. Eur J Phycol 35:75–82
Hanagata N, Dubinsky Z (1999) Secondary carotenoid accumulation in Scenedesmus komarekii (Chlorophyceae, Chlorophyta). J Phycol 35:960–966
Hanagata N, Karube I, Chihara M (1996) Bark-inhabiting green algae in Japan (1). Scenedesmus komarekii and Coelastrella multistriata var. multistriata (Scotiellocysteideae, Chlorellaceae, Chlorophyceae). J Jpn Bot 71:87–97
Harker M, Hirschberg J (1997) Biosynthesis of ketocarotenoids in transgenic cyanobacteria expressing algal gene for β-C-4-oxygenase, crtO. FEBS Lett 404:129–134
Harker M, Young AJ (1995) Inhibition of astaxanthin synthesis in the green alga Haematococcus. Eur J Phycol 30:179–187
Hasunuma T, Miyasawa S-I, Yoshimura S, Shinzaki Y, Tomizawa K-I, Shindo K, Choi S-K, Misawa N, Miyake C (2008) Hyperproduction of astaxanthin in plant leaves by transplastomic engineering. Plant J 55:857–868
Higuera-Ciapara I, Felix-Valenzuela L, Goycoolea FM (2006) Astaxanthin: a review of its chemistry and applications. Crit Rev Food Sci Nutr 46:185–196
Hirschberg J (1998) Molecular biology of carotenoid biosynthesis. In: Britton G, Liaaen-Jensen S, Pfander H (eds) Carotenoids vol 3: biosynthesis and metabolism. Birkäuser, Basel, pp 149–194
Hoham RW, Mullet (1977) The life history and ecology of the snow alga Chloromonas cryophila sp. Nov. (Chlorophyta, Volvocales). Phycologia 16:53–68
Hu Z, Li Y, Sommerfeld M, Chen F, Hu Q (2008a) Enhanced protection against oxidative stress in an astaxanthin-overproduction Haematococcus mutant (Chlorophyceae). Eur J Phycol 43:365–376
Hu Q, Sommerfeld M, Jarvis E, Ghirardi M, Posewitz M, Seibert M, Darzins A (2008b) Microalgal triacylglycerols as feedstocks for biofuel production : perspectives and advances. Plant J 54:621–639
Huang JC, Chen F, Sandmann G (2006a) Stress-related differential expression of multiple β-carotene ketolase genes in the unicellular green alga Haematococcus pluvialis. J Biotechnol 122:176–185
Huang JC, Wang Y, Sandmann G, Chen F (2006b) Isolation and characterization of a carotenoid oxygenase gene for Chlorella zofingiensis (Chlorophyta). Appl Microbiol Biotechnol 71:473–479
Hui B, Pei L (2005) An overview of carotenoid production and consumption in China. Carotenoid Sci 9:48
Hui Z, Gongshi X, Xuli D (2005) Advance of natural lutein and astaxanthin in China. Carotenoid Sci 9:49
Huyer G, Liu S, Kelly J, Moffat J, Payette P, Kennedy B, Tsaprailis G, Gresser J, Ramachandran C (1997) Mechanism of inhibition of protein-tyrosine phosphatase by vanadate and pervanadate. J Biol Chem 272:843–851
Imamoglu E, Dalay MC, Sukan FV (2009) Influences of different stress media and high light intensities on accumulation of astaxanthin in the green alga Haematococcus pluvialis. New Biotechnol 26:199–204
Inoue K (2004) Carotenoid hydroxylation—P450 finally! Trends Plant Sci 9:515–517
Ip PF, Chen F (2005a) Employment of reactive oxygen species to enhance astaxanthin formation in Chlorella zofingiensis in heterotrophic culture. Process Biochem 40:3491–3496
Ip PF, Chen F (2005b) Production of astaxanthin by the green alga Chlorella zofingiensis in the dark. Process Biochem 40:733–738
Ip PF, Wong KH, Chen F (2004) Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process Biochem 39:1761–1766
Jang JC, Sheen J (1994) Sugar sensing in higher plants. Plant Cell 6:1665–1679
Jayaraj J, Devlin R, Punja Z (2008) Metabolic engineering of novel ketocarotenoid production in carrot plants. Transgenic Res 17:489–501
Jin E, Lee CG, Polle JEW (2006) Secondary carotenoid accumulation in Haematococcus (Chlorophyceae): biosynthesis, regulation, and biotechnology. J Microbiol Biotechnol 16:821–831
Johnson EA, An GH (1991) Astaxanthin from microbial sources. Crit Rev Biotechnol 11:297–326
Josse EM, Simkin AJ, Gaffé J, Labouré A-M, Kuntz M, Carol P (2000) A plastid terminal oxidase associated with carotenoid desaturation during chromoplast differentiation. Plant Physiol 123:1427–1436
Jyonouchi H, Sun S, Tomita Y, Gross MD (1995) Astaxanthin, a carotenoid without vitamin A activity, augments antibody responses in cultures including T-helper cell clones and suboptimal doses of antigen. J Nutr 125:2483–2492
Kajiwara S, Kakizono T, Saito T, Kondo K, Ohtani T, Nishio N, Nagai S, Misawa N (1995) Isolation and functional identification of a novel cDNA for astaxanthin biosynthesis from Haematococcus pluvialis and astaxanthin synthesis in Escherichia coli. Plant Mol Biol 29:343–352
Kajiwara S, Fraser PD, Kondo K, Misawa N (1997) Expression of an exogenous isopentenyl diphosphate isomerase gene enhances isoprenoid biosynthesis in Escherichia coli. Biochem J 324:421–426
Kakizono T, Kobayashi M, Nagai S (1992) Effect of carbon/nitrogen ratio on encystment accompanied with astaxanthin formation in a green alga, Haematococcus pluvialis. J Ferment Bioeng 74:403–405
Kathiresan S, Chandrashekar A, Ravishankar GA, Sarada R (2009) Agrobacterium-mediated transformation in the green alga Haematococcus pluvialis (Chlorophyceae, Volvocales). J Phycol 45:642–649
Klotz LO (2002) Oxidant-induced signaling: effects of peroxynitrite and singlet oxygen. Biol Chem 383:443–456
Kobayashi M (2003) Astaxanthin biosynthesis enhanced by reactive oxygen species in the green alga Haematococcus pluvialis. Biotechnol Bioprocess Eng 8:322–330
Kobayashi M, Sakamoto Y (1999) Singlet oxygen quenching ability of astaxanthin esters from the green alga Haematococcus pluvialis. Biotechnol Lett 21:265–269
Kobayashi M, Kakizono T, Nishio N, Nagai S (1992) Effects of light intensity, light quality, and illumination cycle on astaxanthin formation in a green alga, Haematococcus pluvialis. J Ferment Bioeng 74:61–63
Kobayashi M, Kakizono T, Nagai S (1993) Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol 59:867–873
Kobayashi M, Kakizono T, Nishio N, Nagai S, Kurimura Y, Tsuji Y (1997a) Antioxidant role of astaxanthin in the green alga Haematococcus pluvialis. Appl Microbiol Biotechnol 48:351–356
Kobayashi M, Kurimura Y, Kakizono T, Nishio N, Tsuji Y (1997b) Morphological changes in the life cycle of the green alga Haematococcus pluvialis. J Ferment Bioeng 84:94–97
Kobayashi M, Todoroki Y, Hirai N, Kurimura Y, Ohigashi H, Tsuji Y (1998) Biological activities of abscisic acid analogs in the morphological change of the green alga Haematococcus pluvialis. J Ferment Bioeng 85:529–531
Komor E, Tanner W (1974) The hexose-proton symport system of Chlorella vulgaris. Specificity, stoichiometry and energetics of sugar-induced proton uptake. Eur J Biochem 44:219–223
Kopecky J, Schoefs B, Stys D, Loest K, Pulz O (2000) Microalgae as a source for secondary carotenoid production. A screening study. Arch Hydrobiol 98:153–167
Ladygin VG (2000) Biosynthesis of carotenoids in the chloroplasts of the green alga Haematococcus pluvialis. Russ J Plant Physiol 47:796–814
Lemaire P, Livingstone DR (1995) Effects of the inhibitor ellipticine on cytochrome P450 reductase and cytochrome P450 (1A1) function in hepatic microsomes of flounder (Platichtys flesus). Mar Environ Res 39:73–77
Lemoine Y, Rmiki N-E, Créach A, Rachidi J, Schoefs B (2008) Cytoplasmic accumulation of astaxanthin by the green alga Haematococcus pluvialis (Flotow). In: Schoefs B (ed) Plant cell compartments—selected topics. Research Signpost, Trivandrum, pp 251–284
Lers A, Levy H, Zamir A (1991) Co-regulation of a gene homologous to early light-induced genes in higher plants and beta-carotene biosynthesis in the alga Dunaliella bardawil. J Biol Chem 266:13698–13705
Levy H, Gokhman I, Zamir A (1992) Regulation and light-harvesting complex II association of a Dunaliella protein homologous to early light-induced proteins in higher plants. J Biol Chem 267:18831–18836
Levy H, Tal T, Shaish A, Zamir A (1993) Cbr, an algal homolog of plant early light-induced proteins, is a putative zeaxanthin binding protein. J Biol Chem 268:20892–20896
Leya T, Rahn A, Lütz C, Remias D (2009) Response of artic snow and permafrost algae to high light and nitrogen stress by changes in pigment composition and applied aspects for biotechnology. FEMS Microbiol Ecol 67:432–443
Li Y, Sommerfeld M, Chen F, Hu Q (2008a) Consumption of oxygen by astaxanthin biosynthesis: a protective mechanism against oxidative stress in Haematococcus pluvialis (Chlorophyceae). J Plant Physiol 165:1783–1797
Li Y, Huang JL, Sandmann G, Chen F (2008b) Glucose sensing and the mitochondria alternative pathway are involved in the regulation of astaxanthin biosynthesis in the dark-grown Chlorella zofingiensis (Chlorophyceae). Planta 228:735–743
Li Y, Huang J, Sandmann G, Chen F (2009) High-light and sodium chloride stress differentially regulate the biosynthesis of astaxanthin in Chlorella zofingiensis (Chlorophyceae). J Phycol 45:635–641
Li Y, Sommerfeld M, Chen F, Hu Q (2010) Effect of photon flux densities on regulation of carotenogenesis and cell viability of Haematococcus pluvialis (Chlorophyceae). J Appl Phycol 22:253–263
Lichtenthaler HK (1999) The 1-deoxy-d-xylulose-5-phosphate pathway of isoprenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 50:47–65
Linden H (1999) Carotenoid hydroxylase from Haematococcus pluvialis: cDNA sequence, regulation and functional complementation. Biochim Biophys Acta 1446:203–212
Liu BH, Lee YK (2000) Secondary carotenoids formation by the green alga Chlorococcum sp. J Appl Phycol 12:301–307
Liu JG, Liu W, Yin MY, Sun YN, Zhang Z, Li YY, Wang ZF, Lin W, Zhang JP, Shi PJ, Zhang XL, Sun YH, Xue YB, Cui XJ (2005) Some scientific and technical approaches to culture Haematococcus pluvialis from laboratory to pilot scale for natural astaxanthin. Carotenoid Sci 9:50
Liu J, Zhong Y, Sun Z, Huang J, Sandmann G, Chen F (2010) One amino acid substitution in phytoene desaturase makes Chlorella zofingiensis resistant to norflurazon and enhances the biosynthesis of astaxanthin. Planta 232:61–67
Lorenz RT, Cysewski GR (2000) Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol 18:160–167
Lotan T, Hirschberg J (1995) Cloning and expression in Escherichia coli of the gene encoding β-C-4-oxygenase, that converts β-carotene to the ketocarotenoid canthaxanthin in Haematococcus pluvialis. FEBS Lett 364:125–128
Lu Y, Jiang P, Liu S, Gan Q, Cui H, Qin S (2010) Methyl jasmonate- or gibberellins A3-induced astaxanthin accumulation is associated with up-regulation of transcription of β-carotene ketolase genes (bkts) in microalga Haematococcus pluvialis. Bioresour Technol 101:6468–6474
Ma RY-N, Chen F (2001) Enhanced production of free trans-astaxanthin by oxidative stress in the cultures of the green microalga. Chlorococcum sp. Process Biochem 36:1175–1179
Mann V, Harker M, Pecker I, Hirschberg J (2000) Metabolic engineering of astaxanthin production in tobacco flowers. Nat Biotechnol 18:888–892
Margalith PZ (1999) Production of ketocarotenoids by microalgae. Appl Microbiol Biotechnol 51:431–438
Martin JF, Gudina E, Barredo JL (2008) Conversion of β-carotene into astaxanthin: two separate enzymes or a bifunctional hydroxylase-ketolase protein? Microb Cell Fact 7:3
Maxwell DP, Nickels R, McIntosh L (2002) Evidence of mitochondrial involvement in the transduction of signals required for the induction of genes associated with pathogen attack and senescence. Plant J 29:269–279
Meng CX, Teng CY, Jiang P, Qin S, Tseng C (2005) Cloning and characterization of β-carotene ketolase gene promoter in Haematococcus pluvialis. Acta Biochim Biophys Sinica 37:270–275
Miao F, Dayan L, Li Y, Zeng M (2006) Characterization of astaxanthin esters in Haematococcus pluvialis by liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. Anal Biochem 352:176–181
Misawa N (2009) Pathway engineering of plants toward astaxanthin production. Plant Biotechnol 26:93–99
Misawa N, Satomi Y, Kondo K, Yokoyama Y, Kajiwara S, Saito T, Ohtani T, Miki W (1995) Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J Bacteriol 177:6575–6584
Mittler R (2002) Oxidative stress, antioxidants and stress tolerance. Trends Plant Sci 7:405–410
Miura Y, Kondo K, Saito T, Shimada H, Fraser PD, Misawa N (1998) Production of the carotenoids lycopene, beta-carotene, and astaxanthin in the food yeast Candida utilis. Appl Environ Microbiol 64:1226–1229
Moller IM (2001) Plant mitochondria and oxidative stress: electron transport, NADPH turnover, and metabolism of reactive oxygen species. Annu Rev Plant Physiol Plant Mol Biol 52:561–591
Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300:332–336
Morris WL, Ducreux LJM, Fraser PD, Millam S, Taylor MA (1986) Engineering of ketocarotenoids in potato tubers. Metab Eng 8:253–263
Moulin P, Lemoine Y, Schoefs B (2010) Modifications of the carotenoid metabolism in plastids: a response to stress conditions. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York; 3rd edn, Taylor and Francis, New York, pp 407–433
Müller T, Bluβ W, Martin CD, Rogaschewski S, Fuhr G (1998) Snow from Northwest Svalbard: their identification, distribution, pigment and nutrient content? Polar Biol 20:14–32
Nebdalova L, Kocianova M, Lukavsky J (2008) Ecology of snow algae in the Giant Mts. Opera Corcon 45:59–68
Niyogi KK (2000) Safety valves for photosynthesis. Curr Opin Plant Biol 3:455–460
Olaizola M (2000) Commercial production of astaxanthin from Haematococcus pluvialis using 25,000-liter outdoor photobioreactors. J Appl Phycol 12:499–506
Olaizola M, Huntley ME (2003) Recent advances in commercial production of astaxanthin from microalgae. In: Fingerman M, Nagabhushanam R (eds) Recent advances in marine biotechnology. Vol. 9: biomaterials and bioprocessing. Science Publishers, Enfield, NH, pp 143–164
Orosa M, Torres E, Fidalgo P, Abalde J (2000) Production and analysis of secondary carotenoids in green algae. J Appl Phycol 12:553–556
Orosa M, Valero JF, Herrero C, Abalde J (2001) Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other green microalgae under N-starvation and high light conditions. Biotechnol Lett 23:1079–1085
Palozza P, Krinsky NI (1992) Astaxanthin and canthaxanthin are potent antioxidants in a membrane model. Arch Biochem Biophys 297:291–295
Park EK, Lee CG (2001) Astaxanthin production by Haematococcus pluvialis under various light intensities and wavelengths. J Microbiol Biotechnol 11:1024–1030
Park J-K, Tran PN, Kim J-D, Hong S-J, Lee CG (2009) Carotenogenesis in Haematococcus lacustris: role of protein tyrosine phosphatases. J Microbiol Biotechnol 19:918–921
Pelah D, Sintov A, Cohen E (2004) The effect of salt stress on the production of canthaxanthin and astaxanthin by Chlorella zofingiensis grown under limited light intensity. World J Microbiol Biotechnol 20:483–486
Pfannschmidt T, Nilsson A, Allen JF (1999) Photosynthetic control of chloroplast gene expression. Nature 397:625–628
Phillips LG, Cowan AK, Rose PD, Logie MRR (1995) Operation of the xanthophyll cycle of non-stressed and stressed cells of Dunaliella salina Teod. in response to diurnal changes in incident irradiation: a correlation with intracellular beta-carotene content. J Plant Physiol 146:547–553
Qin S, Liu G-X, Hu Z-Y (2008) The accumulation and metabolism of astaxanthin in Scenedesmus obliquus (Chlorophyceae). Process Biochem 43:795–802
Qiu B, Li Y (2006) Photosynthetic acclimation and photoprotective mechanism in Haematococcus pluvialis (Chlorophyceae) during the accumulation of secondary carotenoids at elevated irradiation. Phycologia 45:117–126
Rabbani S, Beyer P, Lintig JV, Hugheney P, Kleinig H (1998) Induced β-carotene synthesis driven by triacylglycerol deposition in the unicellular alga Dunaliella bardawil. Plant Physiol 116:1239–1248
Remias D, Lütz-Meindl U, Lütz C (2005) Photosynthesis, pigments and ultrastructure of the alpine snow alga Chlamydomonas nivalis. Eur J Phycol 40:259–268
Remias D, Holzinger A, Lütz C (2009) Physiology, ultrastructure and habitat of the ice alga Mesotaenium berggrenii (Zygnemaphyceae, Chlorophyta) from glaciers in the European Alps. Phycologia 48:302–312
Remias D, Karsten U, Lütz C, Leya T (2010) Physiological and morphological processes in the Alpine snow alga Chloromonas nivalis (Chlorophyceae) during cyst formation. Protoplasma. doi:10:1007/s00709-010-0123-y
Renstrom B, Liaaen-Jensen S (1981) Fatty acid composition of some esterified carotenols. Comp Biochem Physiol 69(Part B):625–627
Renstrom B, Berger H, Liaaen-Jensen S (1981a) Esterified, optical pure (3S, 3′S)-astaxanthin from flowers of Adonis annua. Biochem Syst Ecol 9:249–250
Renstrom B, Borch G, Skulberg OM, Liaaen-Jensen S (1981b) Optical purity of (3S, 3′S)-astaxanthin from Haematococcus pluvialis. Phytochemistry 20:2561–2564
Rezenka T, Nedbalova L, Sigler K, Cepak V (2008) Identification of astaxanthin diglucoside diesters from snow alga Chlamydomonas nivalis by liquid chromatography-atmospheric pressure chemical mass spectrometry. Phytochemistry 69:479–490
Rise M, Cohen E, Vishkautsan M, Cojocaru M, Gottlieb HE, Arad SM (1994) Accumulation of secondary carotenoids in Chlorella zofingiensis. J Plant Physiol 144:287–292
Rmiki NE, Schoefs B, Juricik B, Lemoine Y (1996) Astaxanthin biosynthesis and encystment induced by light stress in a green unicellular alga. In: Schoefs B, Franck F, Aghion J (eds) Biology, biochemistry and molecular biology of photosynthesis. Bull Soc Roy Sci Liège 35:367–368
Rmiki NE, Schoefs B, Lemoine Y (1999) Carotenoids and stress in higher plants and algae. In: Pessarakli M (ed) Handbook of plant and crop stress. Marcel Dekker, New York, pp 465–482
Rohmer M (2010) Methylerythritol phosphate pathway. In: Mander L, Lui H-W (eds) Comprehensive natural products II chemistry and biology, vol 1. Elsevier, Oxford, pp 517–555
Rolland F, Moore B, Sheen J (2002a) Sugar sensing and signaling in plants. Plant Cell 14:S185–S205
Rolland F, Winderickx J, Thevelein JM (2002b) Glucose-sensing mechanisms in eukaryotic cells. Trends Biochem Sci 26:310–317
Ryu JY, Song JY, Lee JM, Jeong SW, Chow WS, Choi SB, Pogson BJ, Park YI (2004) Glucose-induced expression of carotenoid biosynthesis genes in the dark is mediated by cytosolic pH in the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem 279:25320–25325
Salgado APF, Pereira C, Seica RM, Fernandes AP, Flatt PR, Santos RM, Rosario LM, Ramasamy R (1999) Modulation of glucose-induced insulin secretion by cytosolic redox state in clonal beta-cells. Mol Cell Endocrinol 154:79–88
Sandmann G (1994) Carotenoid biosynthesis in microorganisms and plants. Eur J Biochem 223:7–24
Sandmann G, Kuhn M, Böger P (1993) Carotenoids in photosynthesis: protection of D1 degradation in the light. Photosynth Res 35:185–190
Santos MF, Mesquita JF (1984) Ultrastructural study of Haematococcus lacustris (Girod.) Rostafinski (Volvocales): some aspects of carotenogenesis. Cytologia 49:215–228
Sauer N, Tanner W (1989) The hexose carrier from Chlorella. cDNA cloning of a eucaryotic H+-cotransporter. FEBS Lett 259:43–46
Sayed OH, Hegazy AK (1992) Inhibition of secondary carotenoid biosynthesis during degreening of Chlorella fusca (Chlorococcales, Chlorophyta) and implications for growth and survival. Cryptogam Algol 13:181–186
Schoefs B (2003) Chlorophyll and carotenoid analysis in food products. A practical case-by-case view. Trends Analyt Chem 22:335–339
Schoefs B, Rmiki NE, Rachidi J, Lemoine Y (2001) Astaxanthin accumulation in Haematococcus requires a cytochrome P450 hydroxylase and an active synthesis of fatty acids. FEBS Lett 500:125–128
Schroeder WA, Johnson EA (1995) Singlet oxygen and peroxyl radicals regulate carotenoid biosynthesis in Phaffia rhodozyma. J Biol Chem 270:18374–18379
Seddas P, Gianinazzi-Pearson P, Schoefs B, Küster H, Wipf D (2009) Communication and signaling in the plant-fungus symbiosis: the mycorrhiza. In: Baluska F (ed) Plant-environment interactions from behavioral perspective. Springer Verlag, Berlin, pp 45–71
Semenza GL (1999) Perspectives on oxygen sensing. Cell 98:281–284
Sestak Z, Baslerova M (1963) Changes in chlorophylls and carotenoids in ageing culture of green algae as studied by paper chromatography. In: Studies of microalgae and photosynthetic bacteria, Japan. Soc. Plant Physiol. (ed), University of Tokyo, pp 423–429
Seybold A, Goodwin TW (1959) Occurence of astaxanthin in the flower petals of Adonis annua L. Nature 184:1714–1715
Shimada H, Kondo K, Fraser PD, Miura Y, Saito T, Misawa N (1998) Increased carotenoid production by the food yeast Candida utilis through metabolic engineering of the isoprenoid pathway. Appl Environ Microbiol 64:2676–2680
Somanchi A, Mayfield SP (1999) Nuclear-chloroplast signalling. Curr Opin Plant Biol 2:404–409
Stadler R, Wolf K, Hilgarth C, Tanner W, Sauer N (1995) Subcellular localization of the inducible Chlorella HUP1 monosaccharide-H+ symporter and cloning of a coinduced galactose-H+ symporter. Plant Physiol 107:33–41
Staehelin LA, Arntzen CJ (1983) Regulation of chloroplast membrane function: protein phosphorylation changes the spatial organization of membrane components. J Cell Biol 97:1327–1337
Stalberg K, Lindgren O, Ek B, Höglund AS (2003) Synthesis of ketocarotenoids in the seeds of Arabidopsis thaliana. Plant J 36:771–779
Steinbrenner J, Linden H (2001) Regulation of two carotenoid biosynthetic genes coding for phytoene synthase and carotenoid hydroxylase during stress-induced astaxanthin formation in the green alga Haematococcus pluvialis. Plant Physiol 125:810–817
Steinbrenner J, Linden H (2003) Light induction of carotenoid biosynthesis genes in the green alga Haematococcus pluvialis: regulation by photosynthetic redox control. Plant Mol Biol 52:343–356
Steinbrenner J, Sandmann G (2006) Transformation of the green alga Haematococcus pluvialis with a phytoene desaturase for accelerated astaxanthin biosynthesis. Appl Environ Microbiol 72:7477–7484
Sun Z, Cunningham FX, Gantt E (1998) Differential expression of two isopentenyl pyrophosphate isomerases and enhanced carotenoid accumulation in a unicellular Chlorophyte. Proc Natl Acad Sci USA 95:11482–11488
Sun N, Wang Y, Li Y-T, Huang J-C, Chen F (2008) Sugar-based growth, astaxanthin accumulation and carotenogenesis transcription of heterotrophic Chlorella zofingiensis (Chlorophyceae). Process Biochem 43:1288–1292
Susek RE, Chory J (1992) A tale of two genomes: role of a chloroplast signal coordinating nuclear and plastid genome expression. Aust J Plant Physiol 19:387–401
Tan S, Cunningham FX, Youmans M, Grabowski B, Sun Z, Gantt E (1995) Cytochrome f loss in astaxanthin-accumulating red cells of Haematococcus pluvialis (Chlorophyceae): comparison of photosynthetic activity, photosynthetic enzymes, and thylakoid membrane polypeptides in red and green cells. J Phycol 31:897–905
Tanaka T, Morishita Y, Suzui M, Kojima T, Okumura A, Mori H (1994) Chemoprevention of mouse urinary bladder carcinogenesis by the naturally occurring carotenoid astaxanthin. Carcinogenesis 15:15–19
Tanner W (1969) Light-driven active uptake of 3-O-methylglucose via an inducible hexose uptake system of Chlorella. Biochem Biophys Res Commun 36:278–283
Terao J (1989) Antioxidant activity of β-carotene-related carotenoids in solution. Lipids 24:659–661
Tian L, DellaPenna D (2004) Progress in understanding the origin and functions of carotenoid hydroxylases in plants. Arch Biochem Biophys 430:22–29
Tischer J (1936) Uber das Euglenarhodon und andere Carotinoide einer roten Euglene. (Carotinoide der Süβwasseralgen, I.Teil). Hoppe-Seylers Z Physiol Chem 239:257–269
Tjahjono AE, Hayama Y, Kakizono T, Terada Y, Nishio N, Nagai S (1994) Hyper-accumulation of astaxanthin in a green alga Haematococcus pluvialis at elevated temperatures. Biotechnol Lett 16:133–138
Tracey AS, Gresser MJ (1986) Interaction of vanadate with phenol and tyrosine: implications for the effects of vanadate on systems regulated by tyrosine phosphorylation. Proc Natl Acad Sci USA 83:609–613
Tran N-P, Park J-K, Lee C-G (2009) Proteomic analysis of proteins in green alga Haematococcus pluvialis (Chlorophyceae) expressed under combined stress of nitrogen starvation and high irradiance. Enzyme Microb Technol 45:241–246
Vanlerberghe GC, McIntosh L (1997) Alternative oxidase: from gene to function. Annu Rev Plant Physiol Plant Mol Biol 48:703–734
Vechtel B, Eichenberger W, Ruppel G (1992a) Lipid bodies in Eremosphaera viridis De Bary (Chlorophyceae). Plant Cell Physiol 33:41–48
Vechtel B, Pistorius EK, Ruppel HG (1992b) Occurence of secondary carotenoids in PS I complexes isolated from Eremosphaera viridis De Bary (Chlorophyceae). Z Naturforsch 47c:51–56
Vidhyavathi R, Venkatachalam L, Sarada R, Ravishankar GA (2008) Regulation of carotenoid biosynthetic genes expression and carotenoid accumulation in the green alga Haematococcus pluvialis under nutrient stress conditions. J Exp Bot 59:1409–1418
Vidhyavathi R, Sarada R, Rhavishankar GA (2009) Expression of carotenogenic genes and carotenoid production in Haematococcus pluvialis under the influence of carotenoid and fatty acid synthesis inhibitors. Enzyme Microb Technol 45:88–93
von Flotow J (1844) Uber Haematococcus pluvialis. Verh Kais Leopold-Carol Akad Naturf. Nova Acta Acad Caes Leopold-Carolin Nat Curios 20:411–606
Wang Y, Peng J (2008) Growth-associated biosynthesis of astaxanthin in heterotrophic Chlorella zofingiensis (Chlorophyta). World J Microbiol Biotechnol 24:1915–1922
Wang X, Willen R, Wadström T, Andersen LP (2000) Astaxanthin-rich algal meal and vitamin C inhibit Helicobacter pylori infection in BALB/cA mice. Antimicrob Agents Chemother 44:2452–2457
Wang B, Zarka A, Trebst A, Boussiba S (2003) Astaxanthin accumulation in Haematococcus pluvialis (Chlorophyceae) as an active photoprotective process during high irradiance. J Phycol 39:1116–1124
Wang SB, Chen F, Sommerfeld M, Hu Q (2004) Proteomic analysis of molecular response to oxidative stress by the green alga Haematococcus pluvialis (Chlorophyceae). Planta 220:17–29
Wang JX, Sommerfeld M, Hu Q (2009) Occurence and environmental stress responses of two plastid terminal oxidases in Haematococcus pluvialis (Chlorophyceae). Planta 230:191–203
Werck-Reichhart D, Feyereisen R (2000) Cytochromes P450: a success story. Genome Biol 1:1–9
Wu DY, Wright DA, Wetzel C, Voytas DF, Rodermel S (1999) The immutans variegation locus of Arabidopsis defines a mitochondrial alternative oxidase homolog that functions during early chloroplast biogenesis. Plant Cell 11:43–55
Xiong F, Komenda J, Kopecky J, Nedbal LV (1997) Strategies of ultraviolet-B protection in microscopic algae. Physiol Plant 100:378–388
Yong YYR, Lee YK (1991) Do carotenoids play a photoprotective role in the cytoplasm of Haematococcus lacustris (Chlorophyta)? Phycologia 30:257–261
Young AJ, Britton G (1990) Carotenoids and Stress. In: Alscher RG, Cumming JR (eds) Stress responses in plants: adaptation and acclimation mechanisms. Wiley-Liss, New York, pp 87–112
Zhang DH, Lee YK (2001) Two-step process for ketocarotenoid production by a green alga, Chlorococcum sp. Strain MA-1. Appl Microbiol Biotechnol 55:537–540
Zhekisheva M, Boussiba S, Khozin-Goldberg I, Zarka A, Cohen Z (2002) Accumulation of oleic acid in Haematococcus pluvialis (Chlorophyceae) under nitrogen starvation or high light is correlated with that of astaxanthin esters. J Phycol 38:325–331
Zhekisheva M, Zarka A, Khozin-Goldberg I, Cohen Z, Boussiba S (2005) Inhibition of astaxanthin synthesis under high irradiance does not abolish triacylglycerol accumulation in the green alga Haematococcus pluvialis (Chlorophyceae). J Phycol 41:819–826
Zhu JK (2001) Plant cell tolerance. Trends Plant Sci 6:66–71
Zhu C, Naqvi S, Capell T, Christou P (2009) Metabolic engineering of ketocarotenoid biosynthesis in higher plants. Arch Biochem Biophys 483:182–190
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lemoine, Y., Schoefs, B. Secondary ketocarotenoid astaxanthin biosynthesis in algae: a multifunctional response to stress. Photosynth Res 106, 155–177 (2010). https://doi.org/10.1007/s11120-010-9583-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11120-010-9583-3