Abstract
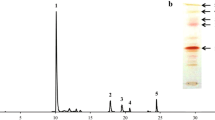
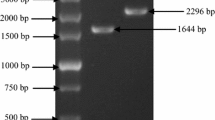
The green alga Chlorella zofingiensis produces large amounts of the valuable ketocarotenoid astaxanthin under dark, heterotrophic growth conditions, making it potentially employable for commercial production of astaxanthin as feed additives, colorants, and health products. Here, we report the identification and characterization of a β-carotene oxygenase (CRTO) gene that is directly involved in the biosynthesis of ketocarotenoids in C. zofingiensis. The open reading frame of the crtO gene, which is interrupted by three introns of 243, 318, and 351 bp, respectively, encodes a polypeptide of 312 amino acid residues. Only one crtO gene was detected in the genome of C. zofingiensis. Furthermore, the expression of the crtO gene was transiently up-regulated upon glucose treatment. Functional complementation in Escherichia coli showed that the coding protein of the crtO gene not only exhibits normal CRTO activity by converting β-carotene to canthaxanthin via echinenone, but also displays a high enzymatic activity of converting zeaxanthin to astaxanthin via adonixanthin. Based on the bifunctional CRTO, a predicted pathway for astaxanthin biosynthesis in C. zofingiensis is described, and the CRTO is termed as carotenoid 4,4′-β-ionone ring oxygenase.





Similar content being viewed by others
References
Albrecht M, Steiger S, Sandmann G (2001) Expression of a ketolase gene mediates the synthesis of canthaxanthin in Synechococcus leading to resistance against pigment photodegradation and UV-B sensitivity of photosynthesis. Photochem Photobiol 73:551–555
Boussiba S (2000) Carotenogenesis in the green alga Haematococcus pluvialis: cellular physiology and stress response. Physiol Plant 108:111–117
Breitenbach J, Misawa N, Kajiwara S, Sandmann G (1996) Expression in Escherichia coli and properties of the carotene ketolase from Haematococcus pluvialis. FEMS Microbiol Lett 140:241–246
Brown JW (1989) A catalogue of splice junction and putative branch point sequences from plant introns. Nucleic Acids Res 14:9549–9559
Cunningham FX, Gantt E (1998) Genes and enzymes of carotenoid biosynthesis in plants. Annu Rev Plant Physiol Plant Mol Biol 49:557–583
Del-Campo JA, Rodríguez H, Moreno J, Vargas MÁ, Rivas J, Guerrero MG (2004) Accumulation of astaxanthin and lutein in Chlorella zofingiensis (Chlorophyta) Appl Microbiol Biotechnol 64:848–854
Fraser PD, Miura Y, Misawa N (1997) In vitro characterization of astaxanthin biosynthetic enzymes. J Biol Chem 272:6128–6135
Fraser PD, Shimada H, Misawa N (1998) Enzymic confirmation of reactions involved in routes to astaxanthin formation, elucidated using a direct substrate in vitro assay. Eur J Biochem 252:229–236
Guerin M, Huntley ME, Olaizola M (2003) Haematococcus astaxanthin: applications for human health and nutrition. Trends Biotechnol 21:210–216
Ip PF, Chen F (2005) Production of astaxanthin by the green microalga Chlorella zofingiensis in the dark. Process Biochem 40:733–738
Ip PF, Wong KH, Chen F (2004) Enhanced production of astaxanthin by the green microalga Chlorella zofingiensis in mixotrophic culture. Process Biochem 39:1761–1766
Johnson EA, An G-H (1991) Astaxanthin from microbial sources. Crit Rev Biotechnol 11:297–326
Johnson EA, Schroeder WA (1995) Microbial carotenoids. Adv Biochem Eng Biotechnol 53:119–178
Kajiwara S, Kakizono T, Saito T, Kondo K, Ohtani T, Nishio N, Nagai S, Misawa N (1995) Isolation and functional identification of a novel cDNA for astaxanthin biosynthesis from Haematococcus pluvialis, and astaxanthin synthesis in Escherichia coli. Plant Mol Biol 29:343–552
Kobayashi M, Kakizono T, Nagai S (1993) Enhanced carotenoid biosynthesis by oxidative stress in acetate-induced cyst cells of a green unicellular alga, Haematococcus pluvialis. Appl Environ Microbiol 59:867–873
Lee PC, Schmidt-Dannert C (2002) Metabolic engineering towards biotechnological production of carotenoids in microorganisms. Appl Microbiol Biotechnol 60:1–11
Linden H (1999) Carotenoid hydroxylase from Haematococcus pluvialis: cDNA sequence, regulation and functional complementation. Biochem Biophys Acta 1446:203–211
Lorenz RT, Cysewski GR (2000) Commercial potential for Haematococcus microalgae as a natural source of astaxanthin. Trends Biotechnol 18:160–167
Lotan T, Hirschberg J (1995) Cloning and expression in Escherichia coli of the gene encoding β-C-4-oxygenase, that converts β-carotene to the ketocarotenoid canthaxanthin in Haematococcus pluvialis. FEBS Lett 364:125–128
Lu F, Vonshak A, Gabbay R, Hirschberg J, Boussiba S (1995) The biosynthetic pathway of astaxanthin in a green alga Haematococcus pluvialis as indicated by inhibition with diphenylamine. Plant Cell Physiol 36:1519–1524
Margalith PZ (1999) Production of ketocarotenoids by microalgae. Appl Microbiol Biotechnol 51:431–438
McCarthy SS, Kobayashi MC, Niyogi KK (2004) White mutants of Chlamydomonas reinhardtii are defective in phytoene synthase. Genetics 168:1249–1257
Misawa N, Satomi Y, Kondo K, Yokoyama A, Kajiwara S, Saito T, Ohtani T, Miki W (1995) Structure and functional analysis of a marine bacterial carotenoid biosynthesis gene cluster and astaxanthin biosynthetic pathway proposed at the gene level. J Bacteriol 177:6575–6584
Orosa M, Valero JF, Herrero C, Abalde J (2001) Comparison of the accumulation of astaxanthin in Haematococcus pluvialis and other microalgae under N-starvation and high light conditions. Biotechnol Lett 23:1079–1085
Rise M, Cohen E, Vishkautsan M, Cojocaru M, Gottlieb HE, Arad S (1994) Pigment and structural changes in Chlorella zofingiensis upon light and nitrogen stress. J plant Physiol 144:287–292
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning: a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory, Cold Spring Harbor
Sandmann G (1994) Carotenoid biosynthesis in microorganisms and plants. Eur J Biochem 223:7–24
Shi XM, Liu HJ, Zhang XW, Chen F (1999) Production of biomass and lutein by Chlorella protothecoides at various glucose concentrations in heterotrophic cultures. Process Biochem 34:341–347
Stadler R, Wolf K, Hilgarth C, Tanner W, Sauer N (1995) Subcellular localization of the inducible Chlorella HUP1 monosaccharide-H+ symporter and cloning of a co-induced galactose-H+ symporter. Plant Physiol 107:33–41
Steiger S, Sandmann G (2004) Cloning of two carotenoid ketolase genes from Nostoc punctiforme for the heterologous production of canthaxanthin and astaxanthin. Biotechnol Lett 26:813–817
Stewart CN, Via LE (1993) A rapid CTAB isolation technique useful for rapid fingerprinting and other PCR application. Biotechniques 14:748–751
Tanner W (1969) Light-driven uptake of 3-O-methylglucose via an inducible hexose uptake system of Chlorella. Biochem Biophys Res Commun 36:278–283
Wolf K, Tanner W, Sauer N (1991) The Chlorella H+/hexose cotransporter gene. Curr Genet 19:215–219
Acknowledgements
We are grateful to Dr. K.W. Fan for technical assistance. This work was partially supported by a grant from the Research Grants Council of Hong Kong and the Outstanding Young Researcher Award of the University of Hong Kong, the Frontier Research Grant of the SCSIO, and the Hundred-Talents scheme of Chinese Academy of Science.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huang, JC., Wang, Y., Sandmann, G. et al. Isolation and characterization of a carotenoid oxygenase gene from Chlorella zofingiensis (Chlorophyta). Appl Microbiol Biotechnol 71, 473–479 (2006). https://doi.org/10.1007/s00253-005-0166-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-005-0166-8