Abstract
Aims
Sorghum (Sorghum bicolor) roots release biological nitrification inhibitors (BNIs) to suppress soil nitrification. Presence of NH4 + in the rhizosphere stimulates BNIs release and it is hypothesized to be functionally associated with plasma membrane (PM) H+-ATPase activity. However, whether the H+-ATPase is regulated at the transcriptional level, and if so, which isoforms of the H+-ATPases are involved in BNIs release are not known. Also, it is not clear whether the stimulation on BNIs release from roots is due to NH4 + uptake or its assimilation, which are addressed in this study.
Methods
Root exudates from intact sorghum plants were collected using aerated solutions of NH4 + or methyl-ammonium (MeA); and the BNI-activity release was determined. PM vesicles were isolated from fresh roots using a two-phase partitioning system; and the hydrolytic H+-ATPase activity was determined. All genes encoding PM H+-ATPases were searched in sorghum genome, and their expression in response to NH4 + or MeA were analyzed by quantitative RT-PCR in sorghum roots.
Results
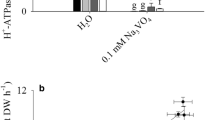
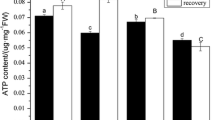
BNIs release and PM H+-ATPase activity increased with NH4 + concentration (≤1.0 mM) in the root-exudate collection solutions, but at higher concentrations, it did not respond further or declined in case of the PM H+-ATPase activity. Twelve PM H+-ATPase genes were identified in sorghum genome; and these isoforms were designated SbA1 to SbA12. Five H+-ATPase genes were stimulated by NH4 + in the rhizosphere, and have similar expression pattern, which is consistent with the variation in H+-ATPase activity. MeA, a non-metabolizable analogue of NH4 +, had no significant effects on BNIs release, H+-ATPase activity, or expression of the H+-ATPase genes.
Conclusions
Our results suggest that the functional link between PM H+-ATPase activity and BNIs release is evident only at NH4 + levels of ≤1.0 mM in the rhizosphere. The variation in PM H+-ATPase activity by NH4 + is due to transcriptional regulation of five isoforms of the H+-ATPases. The stimulatory effect of NH4 + on BNIs release is functionally associated with NH4 + assimilation and not just with NH4 + uptake alone.







Similar content being viewed by others
References
Alsaadawi I, Al-Uqaili J, Alrubeaa A, Al-Hadithy S (1986) Allelopathic suppression of weed and nitrification by selected cultivars of Sorghum bicolor (L.) moench. J Chem Ecol 12:209–219
Alvarez-Pizarro JC, Gomes-Filho E, Prisco JT, Grossi-De-Sá MF, De Oliveira-Neto OB, Da Rocha Fragoso R (2014) Plasma membrane H+-ATPase in sorghum roots as affected by potassium deficiency and nitrogen sources. Biol Plant 58:507–514
Arango M, Gevaudant F, Oufattole M, Boutry M (2003) The plasma membrane proton pump ATPase: the significance of gene subfamilies. Planta 216:355–365
Axelsen KB, Palmgren MG (2001) Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol 126:696–706
Axelsen K, Venema K, Jahn T, Baunsgaard L, Palmgren M (1999) Molecular dissection of the C-terminal regulatory domain of the plant plasma membrane H+-ATPase AHA2: mapping of residues that when altered give rise to an activated enzyme. Biochemistry 38:7227–7234
Duby G, Boutry M (2009) The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles. Pflugers Arch Eur J Physiol 457:645–655
Ermilova EV, Nikitin MM, Fernández E (2007) Chemotaxis to ammonium/methylammonium in Chlamydomonas reinhardtii: the role of transport systems for ammonium/methylammonium. Planta 226:1323–1332
Furukawa J, Yamaji N, Wang H, Mitani N, Murata Y, Sato K, Katsuhara M, Takeda K, Ma JF (2007) An aluminum-activated citrate transporter in barley. Plant Cell Physiol 48:1081–1091
Iizumi T, Mizumoto M, Nakamura K (1998) A bioluminescence assay using Nitrosomonas europaea for rapid and sensitive detection of nitrification inhibitors. Appl Environ Microbiol 64:3656–3662
Ishiyama K, Kojima S, Takahashi H, Hayakawa T, Yamaya T (2003) Cell type distinct accumulations of mRNA and protein for NADH-dependent glutamate synthase in rice roots in response to the supply of NH4+. Plant Physiol Biochem 41:643–647
Kinoshita T, Shimazaki K (2002) Biochemical evidence for the requirement of 14-3-3 protein binding in activation of the guard-cell plasma membrane H+-ATPase by blue light. Plant Cell Physiol 43:1359–1365
Kosola KR, Bloom AJ (1994) Methylammonium as a transport analog for ammonium in tomato (Lycopersicon esculentum L.). Plant Physiol 105:435–442
Lata JC, Durand J, Lensi R, Abbadie L (1999) Stable coexistence of contrasted nitrification statuses in a wet tropical savanna ecosystem. Funct Ecol 13:762–768
Lata JC, Degrange V, Raynaud X, Maron PA, Lensi R, Abbadie L (2004) Grass populations control nitrification in savanna soils. Funct Ecol 18:605–611
Loqué D, von Wirén N (2004) Regulatory levels for the transport of ammonium in plant roots. J Exp Bot 55:1293–1305
Maudoux O, Batoko H, Oecking C, Gevaert K, Vandekerckhove J, Boutry M, Morsomme P (2000) A plant plasma membrane H+-ATPase expressed in yeast is activated by phosphorylation at its penultimate residue and binding of 14-3-3 regulatory proteins in the absence of fusicoccin. J Biol Chem 275:17762–17770
Moore DRE, Waid JS (1971) The influence of washings of living roots on nitrification. Soil Biol Biochem 3:69–83
Ninnemann O, Jauniaux JC, Frommer WB (1994) Identification of a high affinity NH4+ transporter from plants. EMBO J 13:3464–3471
Olsson A, Svennelid F, Ek B, Sommarin M, Larsson C (1998) A phosphothreonine residue at the C-terminal end of the plasma membrane H+-ATPase is protected by fusicoccin-induced 14-3-3 binding. Plant Physiol 118:551–555
O’Neill SD, Spanswick RM (1984) Effects of vanadate on the plasma membrane ATPase of red beet and corn. Plant Physiol 75:586–591
Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Biol 52:817–845
Palmgren MG, Harper JF (1999) Pumping with plant P-type ATPases. J Exp Bot 50:883–893
Palmgren M, Sommarin M, Serrano R, Larsson C (1991) Identification of an autoinhibitory domain in the C-terminal region of the plant plasma membrane H+-ATPase. J Biol Chem 266:20470–20475
Portillo F (2000) Regulation of plasma membrane H+-ATPase in fungi and plants. Biochim Biophys Acta 1469:31–42
Schubert S, Yan F (1997) Nitrate and ammonium nutrition of plants: effects on acid/base balance and adaptation of root cell plasmalemma H+-ATPase. Z Panzenphysiol Bodenkd 160:275–281
Shen H, He LF, Sasaki T, Yamamoto Y, Zheng SJ, Ligaba A, Yan XL, Ahn SJ, Yamaguchi M, Sasakawa H (2005) Citrate secretion coupled with the modulation of soybean root tip under aluminum stress. Up-regulation of transcription, translation, and threonine-oriented phosphorylation of plasma membrane H+-ATPase. Plant Physiol 138:287–296
Subbarao GV, Ishikawa T, Ito O, Nakahara K, Wang HY, Berry WL (2006) A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant Soil 288:101–112
Subbarao GV, Rondon M, Ito O, Ishikawa T, Rao IM, Nakahara K, Lascano C, Berry WL (2007a) Biological nitrification inhibition (BNI)—is it a widespread phenomenon? Plant Soil 294:5–18
Subbarao GV, Wang HY, Ito O, Nakahara K, Berry WL (2007b) NH4 + triggers the synthesis and release of biological nitrification inhibition compounds in Brachiaria humidicola roots. Plant Soil 290:245–257
Subbarao GV, Nakahara K, Hurtado MP, Ono H, Moreta DE, Salcedo AF, Yoshihashi AT, Ishikawa T, Ishitani M, Ohnishi-Kameyama M, Yoshida M, Rondon M, Rao IM, Lascano CE, Berry WL, Ito O (2009) Evidence for biological nitrification inhibition in Brachiaria pastures. Proc Natl Acad Sci U S A 106:17302–17307
Subbarao GV, Sahrawat K, Nakahara K, Ishikawa T, Kishii M, Rao I, Hash C, George T, Srinivasa Rao P, Nardi P (2012a) Biological nitrification inhibition—a novel strategy to regulate nitrification in agricultural systems. Adv Agron 114:249
Subbarao GV, Sahrawat K, Nakahara K, Rao I, Ishitani M, Hash C, Kishii M, Bonnett D, Berry W, Lata J (2012b) A paradigm shift towards low-nitrifying production systems: the role of biological nitrification inhibition (BNI). Ann Bot 112:297–316
Subbarao GV, Nakahara K, Ishikawa T, Ono H, Yoshida M, Yoshihashi T, Zhu Y, Zakir H, Deshpande S, Hash C (2013) Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil 366:243–259
Subbarao GV, Yoshihashi T, Worthington M, Nakahara K, Ando Y, Sahrawat KL, Rao IM, Lata J, Kishii M, Braun H (2015) Suppression of soil nitrification by plants. Plant Sci 233:155–164
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Tesfamariam T, Yoshinaga H, Deshpande SP, Srinivasa Rao P, Sahrawat KL, Ando Y, Nakahara K, Hash CT, Subbarao GV (2014) Biological nitrification inhibition in sorghum: the role of sorgoleone production. Plant Soil 379:325–335
Ullrich WR, Larsson M, Larsson CM, Lesch S, Novacky A (1984) Ammonium uptake in Lemna gibba G 1, related membrane potential changes, and inhibition of anion uptake. Physiol Plant 61:369–376
Van Beusichem ML, Kirkby EA, Baas R (1988) Influence of nitrate and ammonium nutrition on the uptake, assimilation, and distribution of nutrients in Ricinus communis. Plant Physiol 86:914–921
Walker N, Beilby M, Smith F (1979) Amine uniport at the plasmalemma of charophyte cells: I. Current–voltage curves, saturation kinetics, and effects of unstirred layers. J Membr Biol 49:21–55
Xu GH, Fan XR, Miller AJ (2012) Plant nitrogen assimilation and use efficiency. Annu Rev Plant Biol 63:153–182
Yamashita K, Kasai M, Ezaki B, Shibasaka M, Yamamoto Y, Matsumoto H, Sasakawa H (1995) Stimulation of H+ extrusion and plasma membrane H+-ATPase activity of barley roots by ammonium-treatment. Soil Sci Plant Nutr 41:133–140
Yan F, Feuerle R, Schaffer S, Fortmeier H, Schubert S (1998) Adaptation of active proton pumping and plasmalemma H+-ATPase activity of corn roots to low root medium pH. Plant Physiol 117:311–319
Yan F, Zhu Y, Muller C, Zorb C, Schubert S (2002) Adaptation of H+-pumping and plasma membrane H+ ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol 129:50–63
Yuan L, Loque D, Kojima S, Rauch S, Ishiyama K, Inoue E, Takahashi H, von Wiren N (2007) The organization of high-affinity ammonium uptake in Arabidopsis roots depends on the spatial arrangement and biochemical properties of AMT1-type transporters. Plant Cell 19:2636–2652
Zakir HAKM, Subbarao GV, Pearse SJ, Gopalakrishnan S, Ito O, Ishikawa T, Kawano N, Nakahara K, Yoshihashi T, Ono H, Yoshida M (2008) Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (Sorghum bicolor). New Phytol 180:442–451
Zeng HQ, Liu G, Kinoshita T, Zhang RP, Zhu YY, Shen QR, Xu GH (2012) Stimulation of phosphorus uptake by ammonium nutrition involves plasma membrane H+ ATPase in rice roots. Plant Soil 357:205–214
Zeng H, Feng X, Wang B, Zhu Y, Shen Q, Xu G (2013) Citrate exudation induced by aluminum is independent of plasma membrane H+-ATPase activity and coupled with potassium efflux from cluster roots of phosphorus-deficient white lupin. Plant Soil 366:389–400
Zhu YY, Di TJ, Xu GH, Chen X, Zeng HQ, Yan F, Shen QR (2009) Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant Cell Environ 32:1428–1440
Zhu YY, Zeng HQ, Shen QR, Ishikawa T, Subbarao GV (2012) Interplay among NH4 + uptake, rhizosphere pH and plasma membrane H+-ATPase determine the release of BNIs in sorghum roots—possible mechanisms and underlying hypothesis. Plant Soil 358:131–141
Acknowledgments
The research presented here is supported through JIRCAS invitation fellowship program to co-authors (Drs. Houqing Zeng, Tingjun Di and Prof. Yiyong Zhu), and is funded by grant-in-Aid for scientific research from Ministry of Agriculture, Forestry and Fisheries of Japan (MAFF) to JIRCAS under BNI project. Funding support also came from Natural Science Foundation of China (NSFC 31172035) and Program of New Century Excellent Talent in Universities (NCET-11-0672).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Ad C. Borstlap.
Rights and permissions
About this article
Cite this article
Zeng, H., Di, T., Zhu, Y. et al. Transcriptional response of plasma membrane H+-ATPase genes to ammonium nutrition and its functional link to the release of biological nitrification inhibitors from sorghum roots. Plant Soil 398, 301–312 (2016). https://doi.org/10.1007/s11104-015-2675-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-015-2675-2