Abstract
Aims and background
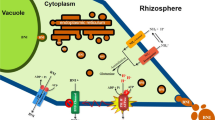
The ability to suppress soil nitrification through the release of nitrification inhibitors from plant roots is termed ‘biological nitrification inhibition’ (BNI). Earlier, we reported that sorghum roots release higher BNI-activity when grown with NH +4 , but not with NO -3 as N source. Also for BNI release, rhizosphere pH of <5.0 is needed; beyond this, a negative effect on BNI release was observed with nearly 80% loss of BNI activity at pH >7.0. This study is aimed at understanding the inter-functional relationships associated with NH +4 uptake, rhizosphere-pH and plasma membrane H+-ATPase (PM H+-ATPase) activity in regulating the release of BNIs (biological nitrification inhibitors) from sorghum roots.
Methods
Sorghum was grown hydroponically and root exudates were collected from intact plants using a pH-stat system to separate the secondary acidification effects by NH +4 uptake on BNIs release. A recombinant luminescent Nitrosomonas europaea bioassay was used to determine BNI-activity. Root plasma membrane was isolated using a two-phase partitioning system. Hydrolytic H+-ATPase activity was determined. Split-root system setup was deployed to understand the localized responses to NH +4 , H+-ATPase-stimulator (fusicoccin) or H+-ATPase-inhibitor (vanadates) on BNI release by sorghum.
Results
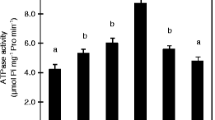
Presence of NH +4 in the rhizosphere stimulated the expression of H+-ATPase activity and enhanced the release of BNIs from sorghum roots. Fusicoccin, which stimulates H+-ATPase activity, also stimulated BNIs release in the absence of NH +4 ; vanadate, which suppresses H+-ATPase activity, also suppressed the release of BNIs. NH +4 levels (in rhizosphere) positively influenced BNIs release and root H+-ATPase activity in the concentration range of 0-1.0 mM, indicating a close relationship between BNI release and root H+-ATPase activity with a possible involvement of carrier-mediated transport for the release of BNIs in sorghum.
Conclusion
Our results suggest that NH +4 uptake, PM H+-ATPase activity, and rhizosphere acidification are functionally inter-connected with BNI release in sorghum. Such knowledge is critical to gain insights into why BNI function is more effective in light-textured, mildly acidic soils compared to other soil types.









Similar content being viewed by others
References
Alsaadawi IS (1988) Biological suppression of nitrification by selected cultivars of Helianthus annuus L. J Chem Ecol 14:733–741
Alsaadawi IS, Al-Uquili JK, Alrubeaa AJ, Al-Hadithy SM (1986) Allelopathic suppression of weed and nitrification by selected cultivars of Sorghum bicolor (L.) Moench. JChem Ecol 12:209–219
Amberger A (1989) Research on dicyandiamide as a nitrification inhibitor and future outlook. Commun Soil Sci Plant Anal 20:1933–1955
Baginski ES, Foa PP, Zak B (1967) Determination of phosphate: study of labile organic phosphate interference. Clinica Chimica Acta 15:155–158
Fillery IRP (2007) Plant-based manipulation of nitrification in soils: a new approach to managing N loss? Plant Soil 294:1–4
Gopalakrishnan S, Watanabe T, Pearse SJ, Ito O, Hossain AZKM, Subbarao GV (2009) Biological nitrification inhibition by Brachiaria humidicola roots varies with soil type and inhibits nitrifying bacteria, but not other major soil microorganisms. Soil Sci Plant Nutr 55:725–733
Hossain AKMZ, Subbarao GV, Pearse SJ, Gopalakrishnan S, Ito O, Ishikawa T, Kawano N, Nakahara K, Yoshihashi T, Ono H, Yoshida M (2008) Detection, isolation and characterization of a root-exuded compound, methyl 3-(4-hydroxyphenyl) propionate, responsible for biological nitrification inhibition by sorghum (Sorghum bicolor). New Phytol 180:442–451
Iizumi T, Nakamura K (1997) Cloning, nucleotide sequence, and regulatory analysis of the Nitrosomonas europaea dnaK gene. Appl Environ Microbiol 63:1777–1784
Iizumi T, Mizumoto M, Nakamura KA (1998) Bioluminescence assay using Nitrosomonas europaea for rapid and sensitive detection of nitrification inhibitors. ApplEnviron Microbiol 64:3656–3662
Larsson C (1985) Plasma membrane. In Modern Methods of Plant Analysis (eds. HF Linskens & JF Jackson), pp. 85–104, Springer-Verlag, Berlin, Germany
Lata JC, Durand J, Lensi R, Abbadie L (1999) Stable coexistence of contrasted nitrification statuses in a wet tropical savanna system. Funct Ecol 13:762–763
Lata JC, Degrange V, Raynaud X, Maron PA, Lensi R, Abbadie L (2004) Grass populations control nitrification in savanna soils. Funct Ecol 18:605–611
Marschner H, Römheld V (1983) In vivo measurement of root induced pH changes at the soil-root interface: effect of plant species and nitrogen source. Zeitschrift für Panzenphysiologie und Bodenkunde 111:241–251
Meinshausen M, Meinshausen N, Hare W, Raper SCB, Frieler K, Knutti R, Frame DJ, Allen MR (2009) Greenhouse-gas emission targets for limiting global warming to 2 C. Nature 458:1158–1162
Mengel K, Robin P, Salsac L (1983) Nitrate reductase activity in shoots and roots of maize seedlings as affected by the form of nitrogen nutrition and the pH of the nutrient solution. Plant Physiol 71:618–622
Mistrik I, Ulrich C (1996) Mechanism of anion uptake in plant roots: quantitative evaluation of H+/NO3- and H+/H2PO4- stoichiometrics. Plant Physiol Biochem 34:629–636
Moorby H, Nye P, White R (1985) The influence of nitrate nutrition on H + efflux by young rape plants (Brassica napus cv. emerald). Plant Soil 84:403–415
Moore DRE, Waid JS (1971) The influence of washing of living roots on nitrification. Soil Biol Biochem 3:69–83
Palmgren MG (2001) Plant plasma membrane H + −ATPase: powerhouses for nutrient uptake. Anu Rev Plant Mol Biol 52:817–845
Palmgren M, Harper J (1999) Pumping with plant P-type ATPases. J Exptl Bot 50:883–893
Parker JH (1972) How fertilizer moves and reacts in soil. Crops Soils 72:7–11
Pearson J, Stewart GR (1993) The deposition of atmospheric ammonia and its effects on plants. New Phytol 125:283–305
Raun WR, Johnson GV (1999) Improving nitrogen use efficiency for cereal production. Agron J 91:357–363
Reid RJ, Field LD, Pitman MG (1985) Effects of external pH, fusicoccin and butyrate on the cytoplasmic pH in barley root tips measured by 31P-nuclear magnetic resonance spectroscopy. Planta 166:341–347
Santi S, Locci G, Monte R, Pinton R, Varanini Z (2003) Induction of nitrate uptake in maize roots: expression of a putative high-affinity nitrate transporter and plasma membrane H+-ATPase isoforms. J Exptl Bot 54:1851–1864
Schubert S, Yan F (1997) Nitrate and ammonium nutrition of plants: effects on acid/base balance and adaptation of root cell plasmalemma H+-ATPase. Zeitschrift für Panzenphysiologie und Bodenkunde 160:275–281
Serrano R (1989) Structure and function of plasma membrane ATPase. Annu Rev Plant Physiol Plant Mol Biol 40:61–94
Slangen J, Kerkhoff P (1984) Nitrification inhibitors in agriculture and horticulture: a literature review. Fertil Res 5:1–76
Smart DR, Bloom AJ (2001) Wheat leaves emit nitrous oxide during nitrate assimilation. Proc Nat Acad Sci (USA) 98:7875–787
Subbarao GV, Ito O, Sahrawat KL, Berry WL, Nakahara K, Ishikawa T, Watanabe T, Suenaga K, Rondon M, Rao IM (2006a) Scope and strategies for regulation of nitrification in agricultural systems – challenges and opportunities. Crit Rev Plant Sci 25:303–335
Subbarao GV, Ishikawa T, Ito O, Nakahara K, Wang HY, Berry WL (2006b) A bioluminescence assay to detect nitrification inhibitors released from plant roots: a case study with Brachiaria humidicola. Plant Soil 288:101–112
Subbarao GV, Rondon M, Ito O, Ishikawa T, Rao IM, Nakahara K, Lascano C, Berry WL (2007a) Biological nitrification inhibition (BNI) - is it a widespread phenomenon? Plant Soil 294:5–18
Subbarao GV, Wang HY, Ito O, Nakahara K, Berry WL (2007b) NH +4 triggers the synthesis and release of biological nitrification inhibition compounds in Brachiara humidicola roots. Plant Soil 290:245–257
Subbarao GV, Ban T, Kishi M, Ito O, Samejima H, Wang HY, Pearse SJ, Gopalakrishnan S, Nakahara K, Hossain AKMZ, Tsujimoto H, Berry WL (2007c) Can biological nitrification inhibition (BNI) genes from perennial Leymus racemosus (Triticeae) combat nitrification in wheat farming? Plant Soil 299:55–64
Subbarao GV, Nakahara K, Ishikawa T, Yoshihashi T, Ito O, Ono H, Ohnishi-Kameyama M, Yoshida M, Kawano N, Berry WL (2008) Free fatty acids from the pasture grass Brachiaria humidicola and one of their methyl esters as inhibitors of nitrification. Plant Soil 313:89–99
Subbarao GV, Nakahara K, Hurtado MP, Ono H, Moreta DE, Salcedo AF, Yoshihashi AT, Ishikawa T, Ishitani M, Ohnishi-Kameyama M, Yoshida M, Rondon M, Rao IM, Lascano CE, Berry WL, Ito O (2009) Evidence for biological nitrification inhibition in Brachiaria pastures. Proc Nat Acad Sci (PNAS) (USA) 106:17302–17307
Subbarao GV, Sahrawat KL, Nakahara K, Ishikawa T, Kishii M, Rao IM, Hash CT, George TS, Srinivasa rao P, Nardi P, Bonnett D, Berry W, Suenaga K, Lata JC (2012a) Biological nitrification inhibition (BNI) – A novel Strategy to regulate nitrification in agricultural systems. Adv Agron 114:249–302
Subbarao GV, Nakahara K, Ishikawa T, Ono H, Yoshida M, Yoshihashi T, Yiyong Zhu, Zakir HAKM, Deshpande SP, Hash CT, Sahrawat KL (2012b) Biological nitrification inhibition (BNI) activity in sorghum and its characterization. Plant Soil (accepted).
Ullrich CI, Novacky A (1990) Extra- and intracellular pH and membrane potential changes induced by K+, Cl-, H2PO -4 and NO -3 uptake and fusicoccin in root hairs of Limnobium stoloniferum. Plant Physiol 94:1561–1567
Wang MY, Siddiqi MY, Ruth TJ, Glass A (1993) Ammonium uptake by rice roots. II. Kinetics of 13NH +4 influx across the plasmalemma. Plant Physiol 103:1259–1267
Wang MY, Glass ADM, Shaff JE, Kochian LV (1994) Ammonium uptake by rice roots. Plant Physiol 104:899–906
Weiske A, Benckiser G, Ottow JCG (2001) Effect of the new nitrification inhibitor DMPP in comparison to DCD on nitrous oxide (N2O) emissions and methane (CH4) oxidation during 3 years of repeated applications in field experiments. Nutr Cycl Agroecosys 60:57–64
Yamashita K, Kasai M, Ezaki B, Shibasaka M, Yamamoto Y, Matsumoto H, Sasakawa H (1995) Stimulation of H+ extrusion and plasma membrane H+-ATPase activity of barley roots by ammonium-treatment. Soil Sci Plant Nutr 41:133–140
Yan F, Zhu Y, Muller C, Zorb C, Schubert S (2002) Adaptation of H+-pumping and plasma membrane H+-ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol 129:50–63
Zerulla W, Barth T, Dressel J, Erhardt K, Von-Locquenghien KH, Pasda G, Radle M, Wissemeier H (2001) 3, 4-Dimethylpyrazole phosphate (DMPP)-a new nitrification inhibitor for agriculture and horticulture. Biol Fertil Soils 34:79–84
Zhu Y, Di T, Xu G, Chen X, Zeng H, Yan F, Shen Q (2009) Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant Cell Environ 32:1428–1440
Acknowledgements
This work was supported by a Grant-in-Aid for scientific research from the Ministry of Agriculture, Forestry and Fisheries of Japan and Natural Science Foundation of China (NSFC 31172035).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Paul Bodelier.
Rights and permissions
About this article
Cite this article
Zhu, Y., Zeng, H., Shen, Q. et al. Interplay among NH +4 uptake, rhizosphere pH and plasma membrane H+-ATPase determine the release of BNIs in sorghum roots – possible mechanisms and underlying hypothesis. Plant Soil 358, 131–141 (2012). https://doi.org/10.1007/s11104-012-1151-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1151-5