Abstract
Aims
Nitrogen, especially NH +4 , can stimulate the uptake of phosphorus in plants, but the underlying mechanisms have not been clearly elucidated. Because phosphate is taken up via an anion/H+ co-transport process, we propose that the stimulated uptake of phosphorus by NH +4 versus NO -3 nutrition may be related to the activity of plasma membrane H+ ATPase. In the present study, we investigated the effect of NH +4 and NO -3 on phosphorus uptake and plasma membrane H+ ATPase activity in rice.
Methods
Rice plants were cultivated in a hydroponic solution with NH +4 or NO -3 . After 15 days of cultivation, phosphorus content was determined. Root plasma membrane was isolated using a two-phase partitioning system and hydrolytic H+-ATPase activity was determined by measuring the Pi concentration after a 30-min hydrolysis reaction. Relative expression of plasma membrane H+ ATPase genes was analyzed by real-time quantitative reverse transcription polymerase chain reaction (qRT-PCR). H+ ATPase enzyme concentration in the plasma membrane was detected by western blot. For 33P uptake experiments, rice roots were incubated in the nutrient solution with addition of H 333 PO4.
Results
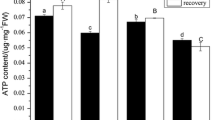
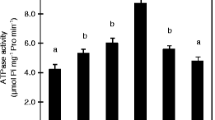
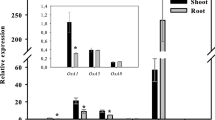
P content in both the roots and shoots of rice plants supplemented with NH +4 was significantly higher than P content in plants grown with NO -3 . Plasma membrane H+ ATPase activity in NH +4 -fed rice roots was significantly higher than that in NO -3 -fed rice roots. Real-time qRT-PCR and western blot results indicated that the higher activity of plasma membrane H+ ATPase in NH +4 -fed rice roots could be attributed to increased expression of the OSA1, OSA3, OSA7, OSA8 and OSA9 genes and an increase in H+ ATPase enzyme concentration in the plasma membrane. Results from 33P uptake experiments showed that rice roots incubated with NH +4 absorbed more 33P during the four-hour incubation than did rice roots incubated with NO -3 . Vanadate inhibited 33P uptake in rice roots supplied with NH +4 , while fusicoccin stimulated 33P uptake under NO -3 nutrition.
Conclusions
Taken together, these results suggest an involvement of plasma membrane H+ ATPase in the stimulated uptake of phosphorus by rice roots supplemented with NH +4 .




Similar content being viewed by others
References
Arango M, Gevaudant F, Oufattole M, Boutry M (2003) The plasma membrane proton pump atpase: the significance of gene subfamilies. Planta 216:355–365
Baginski ES, Foa PP, Zak B (1967) Determination of phosphate: study of labile organic phosphate interference. Clinica Chimica Acta 15:155–158
Bieleski RL (1973) Phosphate pools, phosphate transport, and phosphate availability. Annu Rev Plant Physiol 24:225–252
Blair GJ, Mamaril CP, Miller MH (1971) Influence of nitrogen source on phosphorous uptake by corn from soils differing in pH. Agron J 63:235–238
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Chang CR, Hu YB, Sun SB, Zhu YY, Ma GJ, Xu GH (2009) Proton pump OsA8 is linked to phosphorus uptake and translocation in rice. J Exp Bot 60:557–565
Chiou TJ, Lin SI (2011) Signaling network in sensing phosphate availability in plants. Annu Rev Plant Biol 62:185–206
Daram P, Brunner S, Persson BL, Amrhein N, Bucher M (1998) Functional analysis and cell-specific expression of phosphate transporter from tomato. Planta 206:225–233
Duan YH, Zhang YL, Ye LT, Fan XR, Xu GH, Shen QR (2007) Responses of rice cultivars with different nitrogen use efficiency to partial nitrate nutrition. Ann Bot 99:1153–1160
Duby G, Boutry M (2009) The plant plasma membrane proton pump ATPase: a highly regulated P-type ATPase with multiple physiological roles. Pflugers Archiev – European. J Physiol 457:645–655
Falkengren-Grerup U, Mansson KF, Olsson MO (2000) Uptake capacity of amino acids by ten grasses and forbs in relation to soil acidity and nitrogen availability. Environ Exp Bot 44:207–219
Gahoonia TS, Claassen N, Jungk A (1992) Mobilization of phosphate in different soils by ryegrass supplied with ammonium or nitrate. Plant Soil 140:241–248
Grunes DL (1959) Effect of nitrogen on the availability of soil and fertilizer phosphorous to plants. Adv Agron 11:369–396
Hayashi Y, Nakamura S, Takemiya A, Takahashi Y, Shimazaki K, Kinoshita T (2010) Biochemical characterization of in vitro phosphorylation and dephosphorylation of the plasma membrane H+-ATPase. Plant Cell Physiol 51:1186–1196
Hinsinger P (2001) Bioavailability of soil inorganic P in the rhizosphere as affected by root-induced chemical changes: a review. Plant Soil 237:173–195
Hoffmann C, Ladewig E, Claassen N, Jungk A (1994) Phosphorus uptake of maize as by affected ammonium or nitrate nitrogen-measurements and model calculations. Z Pflanzenernahr Bodenk 157:225–232
Jia H, Ren H, Gu M, Zhao J, Sun S, Zhang X, Chen J, Wu P, Xu G (2011) The phosphate transporter gene OsPht1;8 is involved in phosphate homeostasis in rice. Plant Physiol 156:1164–1175
Jing J, Rui Y, Zhang F, Rengel Z, Shen J (2010) Localized application of phosphorus and ammonium improves growth of maize seedlings by stimulating root proliferation and rhizosphere acidification. Field Crops Res 119:355–364
Kant S, Peng M, Rothstein SJ (2011) Genetic regulation by NLA and microRNA827 for maintaining nitrate-dependent phosphate homeostasis in Arabidopsis. PLoS Genetics 7:e1002021
Kinoshita T, Hayashi Y (2011) New insights into the regulation of stomatal opening by blue light and plasma membrane H+-ATPase. Int Rev Cell Mol Biol 289:89–115
Kronzucker HJ, Siddiqi MY, Glass ADM (1997) Conifer root discrimination against soil nitrate and the ecology of forest succession. Nature 385:59–61
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680–685
Lamaze T, Sentenac H, Grignon C (1984) Effects of nitrate on phosphate accumulation and transport by corn roots. Physiol Veg 22:155–161
Larsson C (1985) Plasma membrane. In: Linskens HF, Jackson JF (eds) Modern methods of plant analysis. Springer-Verlag, Berlin, pp 85–104
Li B, Wang S, Feng H, Xu G (2008) Effects of nitrogen forms on root morphology and phosphate uptake in rice. Chinese J Rice Sci 22(5):665–668 (in Chinese)
Marschner H (1995) Mineral nutrition of higher plants, 2nd edn. Academic, London, pp 231–254
Marschner H, Römheld V (1983) In vivo measurement of root induced pH changes at the soil-root interface: effect of plant species and nitrogen source. Zeitschrift für Panzenphysiologie und Bodenkunde 111:241–251
Mengel K, Schubert S (1985) Active extrusion of protons into deionized water by roots of intact maize plants. Plant Physiol 79:344–348
Miller MH (1974) Effect of nitrogen on phosphorus absorption by plants. In: Carson W (ed) The plant root and its environment. Univ. Press of Virginia, Charlottesville, pp 643–668
Mimura T (1999) Regulation of phosphate transport and homeostasis in plant cells. Int Rev Cytol 191:149–200
Mistrik I, Ullrich C (1996) Mechanism of anion uptake in plant roots: quantitative evaluation of H+/NO -3 and H+/H2PO -4 stoichiometries. Plant Physiol Biochem 34:629–636
Mitsukawa N, Okumura S, Shirano Y, Sato S, Kato T, Harashima S, Shibata D (1997) Overexpression of an Arabidopsis thaliana high-affinity phosphate transporter gene in tobacco cultured cells enhances cell growth under phosphate-limited conditions. Proc Natl Acad Sci U S A 94:7098–7102
Moorby H, Nye P, White R (1985) The influence of nitrate nutrition on H+ efflux by young rape plants (Brassica napus cv. emerald). Plant Soil 84:403–415
Ortas I, Harris EJ, Rowell DL (1996) Enhanced uptake of phosphorus by mycorrhizal sorghum plants as influenced by forms of nitrogen. Plant Soil 184:255–264
Palmgren MG (1998) Proton gradient and plant growth: role of the plasma membrane H+-ATPase. Adv Bot Res 28:1–70
Palmgren M, Harper J (1999) Pumping with plant P-type ATPases. J Exp Bot 50:883–893
Preuss CP, Huang CY, Tyerman SD (2011) Proton-coupled high-affinity phosphate transport revealed from heterologous characterization in Xenopus of barley-root plasma membrane transporter, HvPHT1;1. Plant Cell Environ 34:681–689
Rae AL, Cybinski DH, Jarmey JM, Smith FW (2003) Characterization of two phosphate transporters frombarley; evidence for diverse function and kinetic properties among members of the Pht1 family. Plant Mol Biol 53:27–36
Raghothama KG, Karthikeyan AS (2005) Phosphate acquisition. Plant Soil 274:37–49
Richardson AE (2009) Regulating the phosphorus nutrition of plants: molecular biology meeting agronomic needs. Plant Soil 322:17–24
Riley D, Barber SA (1971) Effect of ammonium and nitrate fertilization on phosphorous uptake as related to root-induced pH changes at the root-soil interface. Soil Sci Soc Am 35:301–306
Sakano K, Yazaki Y, Mimura T (1992) Cytoplasmic acidification induced by inorganic phosphate uptake in suspension cultured Catharanthus roseus cells. Plant Physiol 99:672–680
Sarkar AN, Wyn Jones RGW (1982) Influence of rhizosphere on the nutrient status of dwarf French beans. Plant Soil 64:369–380
Schachtman DP, Reid RJ, Ayling SM (1998) Phosphorus uptake by plants: from soil to cell. Plant Physiol 116:447–453
Schaller G, Fischer WR (1985) pH-Änderungen in der Rhizoshpare von Mais und Erdnuswurzeln. Zeitschrift für Panzenphysiologie und Bodenkunde 148:306–320
Schubert S, Yan F (1997) Nitrate and ammonium nutrition of plants: effects on acid/base balance and adaptation of root cell plasmalemma H+-ATPase. Zeitschrift für Panzenphysiologie und Bodenkunde 160:275–281
Serrano R (1989) Structure and function of plasma membrane ATPase. Annu Rev Plant Physiol Plant Mol Biol 40:61–94
Shen H, Chen J, Wang Z, Yang C, Sasaki T, Yamamoto Y, Matsumoto H, Yan X (2006) Root plasma membrane H+-ATPase is involved in the adaptation of soybean to phosphorus starvation. J Exp Bot 57:1353–1362
Smith FW (2001) Sulphur and phosphorus transport systems in plants. Plant Soil 232:109–118
Smith FW, Jackson WA (1987) Nitrogen enhancement of phosphate transport in roots of Zea mays L. I. Effects of ammonium and nitrate pretreatment. Plant Physiol 84:1314–1318
Song W, Makeen K, Wang D, Zhang C, Xu Y, Zhao H, Tu E, Zhang Y, Shen Q, Xu G (2011) Nitrate supply affects root growth differentially in two rice cultivars differing in nitrogen use efficiency. Plant Soil 343:357–368
Sze H, Li X, Palmgren MG (1999) Energization of plant cell membranes by H+-pumping ATPases: regulation and biosynthesis. Plant Cell 11:677–689
Thien SJ, Mcfee WW (1972) Effect of nitrogen on phosphorous transport systems in Zea mays L. Proc Soil Sci Soc Am 36:617–620
Ullrich CI, Novacky AJ, Fisher E, Liittge U (1981) Relationship between energy-dependent phosphate-uptake and the electrical membrane-potential in Lemna gibba L. Plant Physiol 67:797–801
Ullrich CI, Novacky AJ, van Bell AJE (1984) Phosphate uptake in Lemna gibba G1: energetics and kinetics. Planta 161:45–52
Wang MY, Siddiqi MY, Ruth TJ, Glass A (1993) Ammonium uptake by rice roots. II. Kinetics of 13NH +4 influx across the plasmalemma. Plant Physiol 103:1259–1267
Wiersum LK (1958) Density of root branching as affected by substrate and separate ions. Acta Bot Neerl 7:174–190
Yamaya T, Oaks A (2004) Metabolic regulation of ammonium uptake and assimilation. In: Amancio S, Stulen I (eds) Nitrogen Acquisition and Assimilation in Higher Plants (Plant Ecophysiology Series) (Kluwer Academic Publisher, Dordrecht, the Netherlands, pp 35–64
Yan F, Zhu Y, Muller C, Zorb C, Schubert S (2002) Adaptation of H+-pumping and plasma membrane H+-ATPase activity in proteoid roots of white lupin under phosphate deficiency. Plant Physiol 129:50–63
Zhang R, Liu G, Wu N, Gu M, Zeng H, Zhu Y, Xu G (2011) Adaptation of plasma membrane H+ ATPase and H+ pump to P deficiency in rice roots. Plant Soil 349:3–11
Zhu Y, Di T, Xu G, Chen X, Zeng H, Yan F, Shen Q (2009) Adaptation of plasma membrane H+-ATPase of rice roots to low pH as related to ammonium nutrition. Plant Cell Environ 32:1428–1440
Zhu Y, Lian J, Zeng H, Liu G, Di T, Shen Q, Xu G (2011) Involvement of plasma membrane H+ ATPase in the adaption of rice to ammonium nutrient. Rice Science 18:335–342
Acknowledgements
This work was supported by Natural Science Foundation of China (NSFC 30971864).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Michael Denis Cramer.
Rights and permissions
About this article
Cite this article
Zeng, H., Liu, G., Kinoshita, T. et al. Stimulation of phosphorus uptake by ammonium nutrition involves plasma membrane H+ ATPase in rice roots. Plant Soil 357, 205–214 (2012). https://doi.org/10.1007/s11104-012-1136-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1136-4