Abstract
Aims
Although many studies on the mechanism of Al toxicity and tolerance have been conducted independently, events occurring during the recovery process from Al injury is limited. This study was to investigate Al toxicity recovery mechanism focusing in morphological and physiological aspect.
Methods
We investigated the mechanisms underlying Al toxicity recovery in terms of oxidative stress using the pea root apex as a model system.
Results
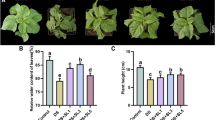
The accumulation of reactive oxygen species was remarkably high in the root under continued Al treatment but decreased in the recovering root. The superoxide anion exuded in the presence of nicotinamide adenine dinucleotide phosphate (NADPH) showed a similar tendency with respect to the accumulation of reactive oxygen species. A similar pattern of lignin content and superoxide dismutase activity was observed among the treatments, while the increased peroxidation in the root under continued Al treatment did not decline with recovery treatment. A longitudinal section of the root under continued Al treatment showed the accumulation of superoxide anion, lignin and peroxide (H2O2) at the epidermal and outer cortex region where the Al induced injuries, including ruptures, are detected.
Conclusions
Oxidative stress is associated with the mechanism of Al toxicity recovery. The recovery process might include the elongation of the central cylinder as a consequence of the oxidative stress-induced formation of the zonal region (ZR). The results further suggest a plausible role for the ZR in the programmed cell death-like function involved in Al toxicity recovery.









Similar content being viewed by others
References
Achary VMM, Jena S, Panda KK, Panda BB (2008) Aluminium induced oxidative stress and DNA damage in root cells of Allium cepa L. Ecotoxicol Environ Saf 70:300–310
Amenós M, Corrale I, Poschnerieder C, Illés P, Baluška F, Barceló J (2009) Different effects of aluminum on the actin cytoskeleton and brefeldin A-sensitive vesicle recycling in root apex cells of two maize varities differeing in root elongation rate and aluminum tolerance. Plant Cell Physiol 50:528–540
Babourina O, Levent O, Cakmak I, Rengel Z (2006) Reactive oxygen species production in wheat roots is not linked with changes in H+ fluxes during acidic and aluminum stresses. Plant Sig Behav 1:70–75
Barcelö AR (1998) Hydrogen peroxide production is a general property of the lignifying xylem from vascular plants. Ann Bot 82:97–103
Basu U, Good A, Taylor GJ (2001) Transgenic Brassica napus plants overexpressing aluminium-induced mitochondrial manganese superoxide dismutase cDNA are resistant to aluminium. Plant Cell Envir 24:1269–1278
Boscolo PRS, Menossi M, Jorge R (2003) Aluminum induced oxidative stress in maize. Phytochem 62:181–189
Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and molecular biology of plants. American Society of Plant Physiologist, Rockville
Budíkóvá S, Čiamporová M (1998) Growth and structural responses of maize roots to aluminium stress. In: Tsekos I, Moustakao M (eds) Progress in Botanical Research. Kluwer Academic Publishers, Dordrecht, pp 419–422
Cakmak I, Horst WJ (1991) Effect of aluminium on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max). Physiol Plant 83:463–468
Cakmak I, Marschner H (1988) Enhanced superoxide radical production in roots of zinc-deficient plants. J Exp Bot 39:1449–1460
Chaffai R, Tekitek A, Ferjani E (2005) Aluminum toxicity in maize seedlings (Zea mays L.): effect on growth and lipid content. J Agron 4:67–74
Čiamporová M (2000) Diverse responses of root cell structure to aluminum stress. Plant Soil 226:113–116
Čiamporová M (2002) Morphological and structural response of plant roots to aluminum at organ, tissue and cellular levels. Biologia Plantarum 45:161–171
Delhaize E, Ryan PR (1995) Aluminum toxicity and tolerance in plants. Plant Physiol 107:315–321
Delisle G, Champoux M, Houde M (2001) Characterization of oxalate oxidase and cell death in Al-sensitive and tolerant wheat roots. Plant Cell Physiol 42:324–333
Devi SR, Yamamoto Y, Matsumoto H (2003) An intracellular mechanism of aluminum tolerance associated with high antioxidant status in cultured tobacco cells. J Inorg Biochem 97:59–68
Ezaki B, Kiyohara H, Matsumoto H, Nakashima S (2007) Overexpression of an auxilin-like gene (F9E10.5) can suppress Al uptake in roots of Arabidopsis. J Exp Bot 58:442–446
Foreman J, Demidchlk V, Boyhwell JHF, Mylona P, Mledeman H, Torres MA, Linsteas P, Costa S, Brownlee C, Jones JDG, Davles JM, Dolan L (2003) Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature 422:442–446
Frahry G, Scopfer P (2001) NADH-stimulated cyanide-resistant superoxide production in maize coleoptiles analyzed with a tetrazolium-based assay. Planta 212:175–183
Giannopolitis CN, Ries SK (1977) Superoxide dismutases 1. Occurrence in higher plants. Plant Physiol 59:309–314
Huang JW, Pellet DW, Papernick LA, Kochian LV (1996) Aluminum interactions with voltage-dependent calcium transport in plasma membrane vesicles isolated from roots of aluminum-sensitive and tolerant wheat cultivars. Plant Physiol 110:561–569
Jones DL, Blancaflor EB, Kochian LV, Gilroy S (2006) Spatial coordination of aluminium uptake, production of reactive oxygen species, callose production and wall rigidification in maize roots. Plant Cell Environ 29:309–1318
Kawano T, Kadono T, Furuichi T, Muto S, Lapeyrie F (2003) Aluminum-induced distortion in calcium signaling involving oxidative burst and channel regulation in tobacco BT-2 cells. Biochem Biophys Res Commun 308:35–42
Kikui S, Sasaki T, Osawa H, Matsumoto H, Yamamot Y (2007) Malate enhances recovery from aluminum-caused inhibition of root elongation in wheat. Plant Soil 290:1–15
Kochian LV, Pineros MA, Hoekenga OA (2005) The physiology, genetics and molecular biology of plant aluminum resistance and toxicity. Plant Soil 274:175–195
Kopittke PM, Blamey FPC, Menzies NW (2008) Toxicities of soluble Al, Cu. and La include rupture on rhizodermal and root critical cells of cowpea. Plant Soil 303:217–227
Li-Juan Q, Bo Z, Shi W-W, Li H-Y (2008) Hydrogen peroxide in planst: a versatile molecule of the reactive oxygen species nrtwork. J Integr Plant Biol 50:2–18
Liszkay A, Kenk B, Schopfer P (2003) Evidence for the involvement of cell wall peroxidase in the generation of hydroxyl radicals mediating extention growth. Planta 217:658–667
Ma B, Wan J, Shen Z (2007) H2O2 production and antioxidant responses in seeds and early seedlings of two different rice varieties exposed to aluminum. Plant Growth Regul 52:91–100
Matsumoto H (2000) Cell biology of aluminum toxicity and tolerance in higher plant. Int Rev Cytol 200:1–46
Matsumoto H, Motoda H (2012) Review Aluminum toxicity recovery processes in root apexes. Possible association with oxidative stress. Plant Sci 185-186:1–8. doi:10.1016/j.plantsci.2011.07.019
Matsumoto H, Yamamoto Y (in press) Plant roots under aluminum stress: Toxicity and tolerance. In Plant Roots: The Hidden Half 4th Edition, Amran E and Beeckman T (ed) Taylor and Francis Books, ISBN: 978-1-4398-4648-3
Mäder M, Amberg-Fisher V (1982) Role of peroxidase in lignifications of tobacco cells 1. Oxidation of nicotinamide adenine dinucleotide and formation of hydrogen peroxide by cell wall peroxidases. Plant Physiol 70:1128–1131
Motoda H, Kano Y, Hiragami F, Kawamura K, Matsumoto H (2010) Morphological changes in the apex of pea roots during and after recovery from aluminum treatment. Plant Soil 33:49–58
Motoda H, Kano Y, Hiragami F, Kawamura K, Matsumoto H (2011) Changes in rupture formation and zonary region stained with Evans blue during the recovery process from aluminum toxicity in the pea root apex. Plant Sig Behav 6:98–100
Navascuis J, P-Rontomé C, Sánchez DH, Staudinger C, Wiankoop S, R-Áivarez R, Becana M (2011) Oxidative stress is a consequence, not a cause, of aluminum toxicity in the forage legume Lotus comiculatus. New Phytol. doi:10.1111/j.1469-8137.2011.03978.X
Ogawa K, Kanematsu S, Asada K (1997) Generation of superoxide anion and localization of Cu Zn-superoxide dismutase in the vascular tissue of spinach hypocotyls: their association with lignifications. Plant Cell Physiol 38:1118–1126
Pan JW, Zhu MY, Chen H (2001) Aluminum-induced cell death in root-tip cells of barley. Envir Exp Bot 46:71–79
Panda SK, Matsumoto H (2010) Changes in antioxidant gene expression and induction of oxidative stress in pea (Pisum sativum L.) under Al stress. Biometals 23:753–762
Rincón-Zachary M, Teacter ND, Sparks JA, Valster AH, Motes CM, Blancaflor B (2010) Fluorescence reasonance energy transfer-sensitized emission of yellow camereon 3.60 reveals root zone-specific calcium signatures in Arabidopsis in response to aluminum and other trivalent cations. Plant Physiol 152:1442–1458
Rodríguez AA, Lascano HR, Bustos D, Taleisnik E (2007) Salinity-induced decrease in NADPH oxidase activity in the maize leaf blade elongation zone. J Plant Physiol 164:223–230
Sagi M, Fluh R (2001) Superoxide production by plant homologues of the gp91phox NADPH oxidase. Modulation of activity by calcium and by tobacco mosaic virus infection. Plant Physiol 126:1281–1290
Sakihama Y, Yamasaki H (2002) Lipid peroxidation induced by phenolics in conjunction with aluminum ions. Biologia Plantarum 45:249–254
Sasaki M, Yamamoto Y, Matsumoto H (1996) Lignin deposition induced by aluminum in wheat (Triticum aestivum) roots. Physiol Plant 96:193–198
Schofield RMS, Pallon J, Fiskesjö G, Karlsson G, Malmqvist KG (1998) Aluminum and calcium distribution patterns in aluminum-intoxicated roots of Allium cepa do not support the calcium-displacement hypothesis and indicate signal-mediater inhibition of root growth. Planta 205:175–180
Tabuchi A, Matsumoto H (2001) Changes in cell-wall properties of wheat (Triticum aesivum) roots during aluminum-induced growth inhibition. Physiol Plant 112:353–358
Tabuchi A, Kikui S, Matsumoto H (2004) Differential effects of aluminum on osmotic potential and sugar accumulation in the root of Al-resistant and Al-sensitive wheat. Physiol Plant 12:106–112
Tahara K, Yamanoshita T, Norisada M, Hasegawa I, Kashima H, Sasaki S, Kojima K (2008) Aluminum distribution and reactive oxygen species accumulation in root tips of two Melaleuca trees differing in aluminum resistance. Plant Soil 307:167–178
Tamas L, Simonovicova M, Huttova J, Mistrik I (2003) Aluminium stimulated hydrogen peroxide production of germinating barley seeds. Envir Exp Bot 51:281–288
Tamas L, Huttova J, Mistrik I (2004) Inhibition of Al-induced root elongation and enhancement of Al-induced peroxidase in Al-sensitive and Al-resistant barley cultivars are positively correlated. Plant Soil 250:193–200
Yamamoto Y, Hachiya O, Matsumoto H (1997) Oxidative damage to membrane by a combination of aluminum and iron in suspension-cultured tobacco cells. Plant Cell Physiol 38:1333–1339
Yamamoto Y, Kobayashi Y, Matsumoto H (2001) Lipid peroxidation is an early symptom triggered by aluminum, but not the primary cause of elongation inhibition in pea roots. Plant Physiol 125:199–208
Yamamoto Y, Kobayashi Y, Devi SR, Rikiishi S, Matsumoto H (2002) Aluminum toxicity and its production of reactive oxygen species in plant cells. Plant Physiol 128:63–72
Yang S (2006) Possible involvement of NADPH oxidase in lanthanide cation-induced superoxide anion generation in BY-2 cells suspension culture. J Rare Earths 24:243–247
Yin L, Mano J, Wang S, Tsuji W, Tanaka K (2010a) The involvement of lipid peroxide-derived aldehydes in aluminum toxicity of tobacco roots. Plant Physiol 152:1406–1417
Yin L, Wang SS, Eltayeb AE, Uddin MI, Yamamoto Y, Tsuji W, Takeuchi Y, Tanaka K (2010b) Overexpression of dehydroascorbate reductase, but not monodehydroascorbate reductase, confers tolerance to aluminum stress in transgenic tobacco. Planta 231:609–621
Zhang W-H, Rengel Z (1999) Aluminium induces an increase in cytoplasmic calcium in intact wheat root apical cells. Aust J Plant Physiol 26:401–409
Zhang B, Wang X, Li X, Ni Y, Li H (2009) Aluminum uptake and disease resistance in Nicotiana rustica leaves. Ecotoxicol Environ Saf 73:655–663
Zheng SJ, Yang J (2005) Target sites of aluminum phytotoxicity. Biologia Plantarum 49:321–331
Zheng K, Pan JW, Ye L, Fu Y, Peng HZ, Kang B, Pan JH, Sha HH, Wang WZ, Zhu MY (2006) Prograrmmed cell death-involved aluminum toxicity in yeast alleviated by antibiotic members with decreased calcium signals. Plant Physiol 143:1–12
Acknowledgements
This research was financially supported through a grant from the Japanese Ministry of Education, Sports, Science and Technology to H.M [Grand-in-Aid for Scientific Research C (2258007)].
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible Editor: Juan Barceló.
Rights and permissions
About this article
Cite this article
Matsumoto, H., Motoda, H. Oxidative stress is associated with aluminum toxicity recovery in apex of pea root. Plant Soil 363, 399–410 (2013). https://doi.org/10.1007/s11104-012-1396-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11104-012-1396-z