Abstract
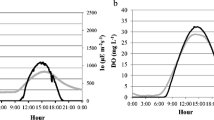
Shallow ponds with rapidly photosynthesising cyanobacteria or eukaryotic algae are used for growing biotechnology feedstock and have been proposed for biofuel production but a credible model to predict the productivity of a column of phytoplankton in such ponds is lacking. Oxygen electrodes and Pulse Amplitude Modulation (PAM) fluorometer technology were used to measure gross photosynthesis (P G) vs. irradiance (E) curves (P G vs. E curves) in Chlorella (chlorophyta), Dunaliella salina (chlorophyta) and Phaeodactylum (bacillariophyta). P G vs. E curves were fitted to the waiting-in-line function [P G = (P Gmax × E/Eopt) × exp(1 — E/Eopt)]. Attenuation of incident light with depth could then be used to model P G vs. E curves to describe P G vs. depth in pond cultures of uniformly distributed planktonic algae. Respiratory data (by O2-electrode) allowed net photosynthesis (P N) of algal ponds to be modelled with depth. Photoinhibition of photosynthesis at the pond surface reduced P N of the water column. Calculated optimum depths for the algal ponds were: Phaeodactylum, 63 mm; Dunaliella, 71 mm and Chlorella, 87 mm. Irradiance at this depth is ≈ 5 to 10 μmol m−2 s−1 photosynthetic photon flux density (PPFD). This knowledge can then be used to optimise the pond depth. The total net P N [μmol(O2) m−2 s−1] were: Chlorella, ≈ 12.6 ± 0.76; Dunaliella, ≈ 6.5 ± 0.41; Phaeodactylum ≈ 6.1 ± 0.35. Snell’s and Fresnel’s laws were used to correct irradiance for reflection and refraction and thus estimate the time course of P N over the course of a day taking into account respiration during the day and at night. The optimum P N of a pond adjusted to be of optimal depth (0.1–0.5 m) should be approximately constant because increasing the cell density will proportionally reduce the optimum depth of the pond and vice versa. Net photosynthesis for an optimised pond located at the tropic of Cancer would be [in t(C) ha−1 y−1]: Chlorella, ≈ 14.1 ± 0.66; Dunaliella, ≈ 5.48 ± 0.39; Phaeodactylum, ≈ 6.58 ± 0.42 but such calculations do not take weather, such as cloud cover, and temperature, into account.
Similar content being viewed by others
Abbreviations
- Chl:
-
chlorophyll
- E:
-
irradiance 400–700 nm photosynthetic photon flux density (PPFD)
- E0 :
-
irradiance at a pond surface
- Eopt :
-
optimum irradiance
- Ex :
-
irradiance at a depth x in a pond
- kL :
-
attenuation constant
- PPFD:
-
photosynthetic photon flux density
- P G :
-
gross photosynthesis expressed on an oxygen basis (P G = rETR/4)
- P N :
-
net photosynthesis (P N = P G + R)
- R :
-
respiration
- rETR:
-
relative electron transport rate
- ΦPSII :
-
effective quantum yield
References
Ai, W., Guo, S., Qin, L., Tang, Y.: Development of a groundbased space micro-algae photo-bioreactor. — Adv. Space Res. 41: 742–747, 2008.
Allen, M.M.: Methods for cyanophyceae. — In: Stein, J.R. (ed.) Handbook of Phycological Methods: Culture Methods and Growth Measurements. Pp. 127–138. Cambridge Univ. Press, Cambridge 1973.
Antoni, D., Zverlov, V.V., Scharz, W.H.: Biofuels from microbes. — Appl. Microbiol. Biotechnol. 77: 23–35, 2007.
Belasco, W.: Algae Burgers for a Hungry World? The Rise and Fall of Chlorella Cuisine. — Technol. Cult. 38: 608–634, 1997.
Behrenfeld, M.J., Falkowski, P.G.: Photosynthetic rates derived from satellite-based chlorophyll concentration. — Limnol. Oceanogr. 42: 1–20, 1997.
Bidigare, R.R., Prezelin, B.B., Smith, R.C.: Bio-optical models and the problems of scaling. — In: Falkowski, P.G. (ed.): Primary Productivity and Biogeochemical Cycles in the Sea. Pp. 175–212. Plenum Press, New York 1992.
Björkman, O., Demmig, B.: Photon yield of O2 evolution and chlorophyll fluorescence characteristics at 77K among vascular plants of diverse origins. — Planta 170: 489–504, 1987.
Borowitzka, L.J.: Commercial production of microalgae: ponds, tanks, tubes and fermenters. — J. Biotechnol. 70: 313–321, 1999.
Borowitzka, M.A.: Algal biotechnology products and processes — matching science and economics. — J. Appl. Phycol. 4: 267–279, 1992.
Borowitzka, M.A., Borowitzka, L.J.: Micro-algal Biotechnology. — Cambridge Univ. Press Publ., Cambridge 1988.
Chisti, Y.: Biodiesel from microalgae. — Biotechnol. Adv. 25: 294–306, 2007.
Chisti, Y.: Biodiesel from microalgae beats bioethanol. — Trends Biotechnol. 26: 126–131, 2008a.
Chisti, Y.: Response to Reijnders: Do biofuels from microalgae beat biofuels from terrestrial plants? — Trends Biotechnol. 26: 351–352, 2008b.
Colinvaux, P.A.: Why Big Fierce Animals are Rare: an Ecologist’s Perspective. — Princeton Univ. Press, Princeton 1978.
Cullen J.J., Geider, R.J., Ishizaka, J. et al.: Towards a general description of phytoplankton growth for biogeochemical models. — In: Evans, G.T., Fasham, M.J.R. (ed.): Towards a General Description of Phytoplankton Growth for Biogeochemical Models. Pp. 153–176. NATO ASl Series 1, Vol. 10. Springer, Berlin 1993.
Dauta, A., Devaux, J., Piquemal, F., Boumnich, L.: Growth rate of four freshwater algae in relation to light and temperature. — Hydrobiologia 207: 221–226, 1990.
Davidson, A.T.: Effects of Ultraviolet Radiation on Microalgal Growth, Survival and Production. — In: Rao, D.V.S. (ed.): Algal Cultures Analogues of Blooms and Applications Vol. II. Pp. 715–767. Science Publishers, Enfield 2006.
Duarte, P.: Photosynthesis-Irradiance Relationships in Marine Algae. — In: Rao, D.V.S. (ed.): Algal Cultures Analogues of Blooms and Applications. Vol. II. Pp. 639–670. Science Publishers, Enfield 2006.
Eilers, P.H.C., Peeters, J.C.H.: A model for the relationship between light intensity and the rate of photosynthesis in phytoplankton. — Ecol. Model. 42: 199–215, 1988.
Engqvist, A., Sjöberg, S.: An analytical integration method of computing diurnal primary production from Steele’s light response curve. — Ecol. Model. 8: 219–232, 1980.
Falkowski, P.G.: Light-shade adaptation and assimilation numbers. — Plankton Res. 3: 203–216, 1981.
Falkowski, P.G., Greene, R., Kolber, Z.: Light utilization and photoinhibition of photosynthesis in marine phytoplankton. — In: Baker, N.R., Bowyer, J.R. (ed.): Photoinhibition of Photosynthesis from Molecular Mechanisms to the Field. Pp. 407–432. BIOS Scietific Publ., Oxford 1994.
Falkowski, P.G., Raven, J.A.: Aquatic photosynthesis. 2nd E.n. Princeton Univ. Press, Princeton 2007.
Fawley, M.W.: Effects of light intensity and temperature interactions on growth characteristics of Phaeodactylum tricornutum (Bacillariophyceae). — J. Phycol. 20: 67–72, 1984.
Garcia, H.E., Gordon, L.: Oxygen Solubility in Seawater: Better Fitting Equations. — Limnol. Oceanogr. 37: 1307–1312, 1992.
Genty, B., Briantais, J.M., Baker, N.R.: The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. — Biochim. Biophys. Acta 990: 87–92, 1989.
Giordano, M., Beardall, J.: Impact of environmental conditions on photosynthesis, growth and carbon allocation strategies of hypersaline species of Dunaliella. — Global Nest J. 11: 79–85, 2009.
Gloag, R.S., Ritchie, R.J., Chen, M., Larkum, A.W.D., Quinnell, R.G.: Chromatic photoacclimation, photosynthetic electron transport and oxygen evolution in the Chlorophyll dcontaining oxyphotobacterium Acaryochloris marina Miyashita. — Biochim. Biophys. Acta-Bioenergetics 1767: 127–135, 2007.
Grobbelaar, J.U.: Photosynthetic characteristics of Spirulina platensis grown in commercial-scale open outdoor raceway ponds: what do the organisms tell us? — J. Appl. Phycol. 19: 591–598, 2007.
Grobbelaar, J.U., Soeder, C.J., Stengel, E.: Modelling Algal Productivity in Large Outdoor Cultures and Waste Treatment Systems. — Biomass 21: 297–314, 1990.
Huntley, M.E., Redalje, D.G.: CO2 mitigation and renewable oil from photosynthetic microbes: a new appraisal. — Mitig. Adapt. Strat. GL 12: 573–608, 2007.
Jassby, A.D., Platt T.: Mathematical formulation of the relationship between photosynthesis and light for phytoplankton. — Limnol. Oceanogr. 21: 540–547, 1976.
Johnson, M.L., Faunt, L.M.: Parameter estimation by least squares methods. — Methods Enzymol. 210: 1–37, 1992.
Kroon, B.M.A., Ketelaars, H.A.M., Fallowfield, H.J., Mur, L.R.: Modelling microalgal productivity in a High Rate Algal Pond based on wavelength dependent optical properties. — J. Appl. Phycol. 1: 247–256, 1989.
Larkum, A.W.D.: Limitations and prospects of natural photosynthesis for bioenergy production. — Curr. Opin. Biotechnol. 21: 271–276, 2010.
Larkum, A.W.D., Douglas, S.E., Raven, J.A. (ed.): Photosynthesis in Algae. — Kluwer Academic, Dordrecht 2003.
Larkum, T., Howe, C.J.: Molecular Aspects of Light-harvesting Processes in Algae. — Adv. Bot. Res. 27: 257–330, 1997.
Lorentzen, C.J.: The Penetration of light in the sea. — In: Cushing, D.H., Walsh, J.J. (ed.): The Ecology of the Seas. Pp. 173–185. Blackwell Scientific, Oxford 1976.
Lorrain, P., Corson, D.R., Lorrain, F.: Plane electromagnetic waves III.- In: Lorrain, P., Corson, D.R., Lorrain, F.: Electromagnetic Fields and Waves. Pp. 557–561. Freeman, New York 1988.
MacIntyre, H.L., Kana, T.M., Anning, T., Geider, R.J.: Photoacclimation of photosynthesis irradiance response curves and photosynthetic pigments in microalgae and cyanobacteria. — J. Phycol. 38: 17–38, 2002.
McBride, G.B.: Calculation of Daily Photosynthesis by Means of Five Photosynthesis-Light Equations. — Limnol. Oceanogr. 37: 1796–1808, 1992.
Melis, A.: Spectroscopic methods in photosynthesis: photosystem stoichiometry and chlorophyll antenna size. — Philos. Trans. Roy. Soc. London Ser. B 323: 397–409, 1989.
Miller, C.B.: Biological Oceanography. — Blackwell Publ., Malden 2006.
Moheimani, N.R., Borowitzka, M.A.: The long-term culture of the coccolithophore Pleurochrysis carterae (Haptophyta) in outdoor raceway ponds. — J. Appl. Phycol. 18: 703–712, 2006a.
Moheimani, N.R., Borowitzka, M.A.: Limits to productivity of the alga Pleurochrysis carterae (Haptophyta) grown in outdoor raceway ponds. — Biotechnol. Bioeng. 1: 27–36, 2006b.
Morel, A.: Light and marine photosynthesis: a spectral model with geochemical and climatological implications. — Prog. Oceanogr. 26: 263–306, 1991.
Nishri, A., Ben Yarkov, S.: Solubility of oxygen in the Dead Sea. — Hydrobiologia 197: 99–104, 1990.
Oswald, W.J.: Productivity of algae in sewage disposal. — Sol. Energy 15: 107–117, 1973.
Platt T., Sathyendranath S.: Oceanic primary production: estimation by remote sensing at local and regional scales. — Science 241: 1613–1620, 1988.
Ralph, P.J., Gademann, R.: Rapid light curves: A powerful tool to assess photosynthetic activity. — Aquat. Bot. 82: 222–237, 2005.
Richmond, A., Zou, N.: Efficient utilisation of high photon irradiance for mass production of photoautotrophic microorganisms. — J. Appl. Phycol. 11: 123–127, 1999.
Ritchie, R.J.: Consistent sets of spectrophotometric equations for acetone, methanol and ethanol solvents. — Photosynth. Res. 89: 27–41, 2006.
Ritchie, R.J.: Universal chlorophyll equations for estimating chlorophylls a, b, c and d and total chlorophylls in natural assemblages of photosynthetic organisms using acetone, methanol or ethanol solvents. — Photosynthetica 46: 115–126, 2008a.
Ritchie, R.J.: Fitting light saturation curves measured using PAM fluorometry. — Photosynth. Res. 96: 201–215, 2008b.
Ritchie, R.J.: Modelling Photosynthetically Active Radiation and Maximum Potential Gross Photosynthesis. — Photosynthetica 48: 596–609, 2010.
Ritchie, R.J.: Photosynthesis in the Blue Water Lily (Nymphaea caerulea Saligny) using PAM Fluorometry. — Int. J. Plant Sci. 173: 124–136, 2012.
Ritchie, R.J., Bunthawin, S.: The use of PAM (Pulse Amplitude Modulation) fluorometry to measure photosynthesis in a CAM orchid, Dendrobium spp. (D. ‘Viravuth’ Pink). — Int. J. Plant Sci. 171: 575–585, 2010a.
Ritchie, R.J., Bunthawin, S.: The Use of PAM (Pulse Amplitude Modulation) Fluorometry to Measure Photosynthesis in Pineapple (Ananas comosus [L.] Merr). — Trop. Plant Biol. 3: 193–203, 2010b.
Robertson, M.J., Wood, A.W., Muchow, R.C.: Growth of sugarcane under high input conditions in tropical Australia. I. Radiation use, biomass accumulation and partitioning. — Field Crops Res. 48: 11–25, 1996.
Sheehan, J., Dunahay, T., Benemann, J., Roessler, P.: A look back at the US Department of Energy’s Aquatic Species Program: Biodiesel from Algae. NREL/TP-580-24190. NREL, Golden, Colorado, 1998. (Available at http://www1.eere.energy.gov/biomass/pdfs/biodiesel_from_algae.pdf. [Accessed 03 January 2009].)
Sherwood, J.E., Stagnitti, F., Kokkinn, M.J., Williams, W.D.: Dissolved oxygen concentrations in hypersaline waters. — Limnol. Oceanogr. 36: 235–250, 1991.
Shimamatsu, H.: A pond for edible Spirulina production and its hydraulic studies. — Hydrobiologia 151/152: 83–89, 1987.
Shimamatsu, H.: Mass production of Spirulina, an edible microalga. — Hydrobiologia 512: 39–44, 2004.
SMARTS: Simple Model of Atmospheric Radiative Transfer of Sunshine (SMARTS): http://www.nrel.gov/rredc/smarts/ [Accessed 23/11/2009].
Smayda, T.J.: Autecology of bloom-forming microalgae: extrapolation to field populations and the refield-braarud debate revisited. — In: Rao, D.V.S. (ed.): Algal Cultures Analogues of Blooms and Applications. Vol. I, Pp. 215–270. Science Publishers, Enfield 2006.
Sosik, H.M., Mitchell, B.G.: Effects of temperature on growth, light absorption, and quantum yield in Dunaliella tertiolecta (Chlorophyceae). — J. Phycol. 30: 833–840, 1994.
Steele, J.H.: Environmental control of photosynthesis in the sea. — Limnol. Oceanogr. 7: 137–150, 1962.
Stemke, J.A., Santiago, L.S.: Consequences of light absorptance in calculating electron transport rate of desert and succulent plants. — Photosynthetica 49: 195–200, 2011.
Sukenik, A., Levy, R.S., Levy, Y., Falkowski, P.G., Dubinsky, Z.: Optimizing algal biomass production in an outdoor pond: a simulation model. — J. Appl. Phycol. 3: 191–201, 1991.
Talling, J.F, Wood, R.B, Prosser, M.V., Baxter, R.M.: The upper limit of photosynthetic productivity by phytoplankton: evidence from Ethiopian soda lakes. — Freshwater Biol. 3: 53–76, 1973.
Walker, D.A.: The Use of the Oxygen Electrode and Fluorescence Probes in Simple Measurements of Photosynthesis. — Oxygraphics Publ., Sheffield 1990.
Walker, D.A.: Biofuels, facts, fantasy, and feasibility. — J. Appl. Phycol. 21: 509–517, 2009.
Walker, D.A.: Biofuels — for better or worse? — Ann. Appl. Biol. 156: 319–327, 2010.
Waltz, E.: Biotechs green gold? — Nat. Biotechnol. 27: 15–18, 2009.
Warburg, O.: [On the rate of photochemical carbonic acid decomposition in living cells.] — Biochem. Z. 100: 232–262, 1919. [In German.]
Weissman, J.C., Goebel, R.P., Benemann, J.R.: Photobioreactor design: mixing, carbon utilization, and Oxygen Accumulation. — Biotechnol. Bioeng. 31: 336–344, 1988.
Westlake, D.F.: Comparisons of plant productvity. — Biol. Rev. 38: 385–425, 1963.
Weyer, K.M., Bush, D.R., Darzins, A., Willson, B.D.: Theoretical maximum algal oil production. — Bioenerg. Res. 3: 204–213, 2010.
White, A.J., Critchley, C.: Rapid light curves: A new fluorescence method to assess the state of the photosynthetic apparatus. — Photosynth. Res. 59: 63–72, 1999.
Zmora, O., Richmond, A.: Microalgae for aquaculture, microalgae production for aquaculture. — In: Richmond, A. (ed.): Handbook of Microalgal Culture: Biotechnology and Applied Phycology. Pp. 365–79. Blackwell Scientific, Oxford 2004.
Author information
Authors and Affiliations
Corresponding author
Additional information
Acknowledgements: The authors wish to thank Dr. John W. Runcie (University of Sydney), Prof. Michael Borowitzka (Murdoch University) and Mr. Mark Curran (University of Sydney, retired) for their interest in this study and helpful comments on the paper. One of our referees correctly pointed out to us the significance of dissolved organic carbon (DOC) as a significant loss of fixed carbon in ponds and raceways. Dr. Min Chen (University of Sydney) provided laboratory space for RJR at The University of Sydney. Dr. Tania Prvan (Macquarie University) used Maple® software (Maple 10.04, Maplesoft, a division of Waterloo Maple Inc. 1981–2006) for the solving integral calculus problems necessary in the study. The EXCEL© files for light curve fitting, chlorophyll measurements, calculation of reflection and refraction and solar irradiance and solar angle data (for selected latitudes) are available upon request.
Rights and permissions
About this article
Cite this article
Ritchie, R.J., Larkum, A.W.D. Modelling photosynthesis in shallow algal production ponds. Photosynthetica 50, 481–500 (2012). https://doi.org/10.1007/s11099-012-0076-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11099-012-0076-9