Abstract
Purpose
The purpose of this study was to present a modified Andersen cascade impactor (ACI) as a platform to evaluate the deposition and subsequent transport of aerosol micropaticles across airway epithelial cells.
Methods
The impaction plate of an ACI was modified to accommodate up to eight Snapwells. Aerodynamic particle size distribution of the modified ACI was investigated with two commercially available formulations of Ventolin® (salbutamol sulphate) and QVAR® (beclomethasone dipropionate). Deposition and transport of these drug microparticles across sub-bronchial epithelial Calu-3 cells were also studied.
Results
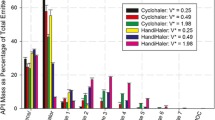
The modified ACI demonstrated reproducible deposition patterns of the commercially available pressurised metered dose inhalers compared to the standard ACI. Furthermore, the Calu-3 cells could be placed in different stages of the modified ACI. No significant effect was observed among the transport rate of different particle sizes deposited on Calu-3 cells within the range of 3.3 to 0.4 μm.
Conclusions
The use of the cell compatible ACI to assess the fate of microparticles after deposition on the respiratory epithelia may allow for better understanding of deposited microparticles in vivo.




Similar content being viewed by others
References
Edwards DA, Hanes J, Caponetti G, Hrkach J, Ben-Jebria A, Eskew ML, et al. Large porous particles for pulmonary drug delivery. Science. 1997;276(5320):1868–72.
Haghi M, Bebawy M, Colombo P, Forbes B, Lewis DA, Salama R, et al. Towards the bioequivalence of pressurised metered dose inhalers 2. Aerodynamically equivalent particles (with and without glycerol) exhibit different biopharmaceutical profiles in vitro. Eur J Pharm Biopharm. 2013. doi:10.1016/j.ejpb.2013.02.020.
Patton JS, Byron PR. Inhaling medicines: delivering drugs to the body through the lungs. Nat Rev Drug Discov. 2007;6(1):67–74.
Bur M, Lehr C-M. Pulmonary cell culture models to study the safety and efficacy of innovative aerosol medicines. Expert Opin Drug Deliv. 2008;5(6):641–52.
Online databese Stationery Office. British Pharmacopoeia, Appendix XII C London. 2012. Accessed 01 June 2013. Available from: http://www.pharmacopoeia.co.uk.
Swift DL. Use of mathematical aerosol deposition models in predicting the distribution of inhaled therapeutic aerosols. In: Hickey AJ, editor. Inhalation aerosols. New York: Marcel Dekker; 1996. p. 51–7.
Davies NM, Feddah MR. A novel method for assessing dissolution of aerosol inhaler products. Int J Pharm. 2003;255(1–2):175–87.
Arora D, Shah K, Halquist M, Sakagami M. In Vitro Aqueous Fluid-Capacity-Limited Dissolution Testing of Respirable Aerosol Drug Particles Generated from Inhaler Products. Pharm Res. 2010;27(5):786–95.
Dunbar C, Mitchell J. Analysis of cascade impactor mass distributions. J Aerosol Med. 2005;18(4):439–51.
Agu RU, Ugwoke MI. In vitro and in vivo testing methods for respiratory drug delivery. Expert Opin Drug Deliv. 2011;8(1):57–69.
Forbes B, Ehrhardt C, Forbes B, Ehrhardt C. Human respiratory epithelial cell culture for drug delivery applications. Eur J Pharm Biopharm. 2005;60(2):193–205.
Grainger CI, Greenwell LL, Martin GP, Forbes B. The permeability of large molecular weight solutes following particle delivery to air-interfaced cells that model the respiratory mucosa. Eur J Pharm Biopharm. 2009;71(2):318–24.
Grainger CI, Saunders M, Buttini F, Telford R, Merolla LL, Martin GP, et al. Critical characteristics for corticosteroid solution metered dose inhaler bioequivalence. Mol Pharm. 2012;9(3):563–9.
Haghi M, Traini D, Bebawy M, Young PM. Deposition, Diffusion and transport mechanism of dry powder microparticulate salbutamol, at the respiratory epithelia. Mol Pharm. 2012;9(6):1717–26.
Haghi M, Salama R, Traini D, Bebawy M, Young P. Modification of disodium cromoglycate passage across lung epithelium in vitro via incorporation into polymeric microparticles. AAPSJ. 2012;14(1):79–86.
Haghi M, Traini D, Postma DS, Bebawy M, Young PM. Mediated fluticasone uptake across Calu-3 cells by salmeterol as combination powder inhaler. Respirology. 2013. doi:10.1111/resp.12146.
Fiegel J, Ehrhardt C, Schaefer UF, Lehr CM, Hanes J. Large porous particle impingement on lung epithelial cell monolayers–toward improved particle characterization in the lung. Pharm Res. 2003;20(5):788–96.
Bur M, Rothen-Rutishauser B, Huwer H, Lehr C-M. A novel cell compatible impingement system to study in vitro drug absorption from dry powder aerosol formulations. Eur J Pharm Biopharm. 2009;72(2):350–7.
Hein S, Bur M, Schaefer UF, Lehr C-M. A new pharmaceutical aerosol deposition device on cell cultures (PADDOCC) to evaluate pulmonary drug absorption for metered dose dry powder formulations. Eur J Pharm Biopharm. 2011;77(1):132–8.
McDonnell C, Shur J, Burns J, Hipkiss D, Price R. Investigation deposition and drug absorption of budesonide microparticles using a cell compatible next generation impactor. Respir Drug Deliv Proc. 2012; Vol 3, pp 785-788. Davis Healthcare Int’l Publishing. Illinois, USA.
Cooney D, Kazantseva M, Hickey AJ. Development of size-dependent aerosol deposition model utilizing human airway epithelial cells for evaluating aerosol Drug Delivery. ALTA. 2004;32(6):581–90.
Exiarch H, Haltner-Ukomadu E, Beisswenger C, Bock U. Drug delivery to the lung: Permeability and physicochemical characteristics of drugs as the basis for a pulmonary biopharmaceutical classification system (pBCS). J Epithelial Biol Pharmacol. 2010;3:1–14.
Haghi M, Young PM, Traini D, Jaiswal R, Gong J, Bebawy M. Time- and passage-dependent characteristics of a Calu-3 respiratory epithelial cell model. Drug Dev Ind Pharm. 2010;36(10):1207–14.
Grainger C, Greenwell L, Lockley D, Martin G, Forbes B. Culture of Calu-3 cells at the air interface provides a representative model of the airway epithelial barrier. Pharm Res. 2006;23(7):1482–90.
Mathias NR, Timoszyk J, Stetsko PI, Megill JR, Smith RL, Wall DA, et al. Permeability characteristics of calu-3 human bronchial epithelial cells: in vitro-in vivo correlation to predict lung absorption in rats. J Drug Target. 2002;10(1):31–40.
Mitchell J, Nagel M, Wiersema K, Doyle C. Aerodynamic particle size analysis of aerosols from pressurized metered-dose inhalers: Comparison of andersen 8-stage cascade impactor, next generation pharmaceutical impactor, and model 3321 aerodynamic particle sizer aerosol spectrometer. AAPS PharmSciTech. 2003;4(4):425–33.
Nagel MW, Wiersema KJ, Bates SL, Mitchell JP. Size analysis of a pressurized metered dose inhaler-delivered solution formulation by an Aerosizer-LD time-of-flight aerosol particle size spectrometer. J Aerosol Med Pulm Drug Deliv. 2002;15(1):75–85.
Hoe S, Young P, Chan H-K, Traini D. Introduction of the Electrical Next Generation Impactor (eNGI) and Investigation of its Capabilities for the Study of Pressurized Metered Dose Inhalers. Pharm Res. 2009;26(2):431–7.
Kotian R, Peart L, Bryner J, Byron PR. Calibration of the modified electrical low-pressure impactor (ELPI) for use with pressurized pharmaceutical aerosols. J Aerosol Med Pulm Drug Deliv. 2009;22(1):55–66.
Bur M, Huwer H, Muys L, Lehr C-M. Drug transport across pulmonary epithelial cell monolayers: Effects of particle size, apical liquid volume, and deposition technique. J Aerosol Med Pulm Drug Deliv. 2010;23(3):119–27.
Lewis DA, Young PM, Buttini F, Church T, Colombo P, Forbes B, et al. Towards the bioequivalence of pressurised metered dose inhalers 1: Design and characterisation of aerodynamically equivalent beclomethasone dipropionate inhalers with and without glycerol as a non-volatile excipient. Eur J Pharm Biopharm. 2013. doi:10.1016/j.ejpb.2013.02.014.
Acknowledgments and Disclosures
A/Professor Traini is the recipient of an Australian Research Council Future Fellowship (project number FT12010063). A/Professor Young is the recipient of an Australian Research Council Future Fellowship (project number FT110100996).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Haghi, M., Traini, D. & Young, P. In Vitro Cell Integrated Impactor Deposition Methodology for the Study of Aerodynamically Relevant Size Fractions from Commercial Pressurised Metered Dose Inhalers. Pharm Res 31, 1779–1787 (2014). https://doi.org/10.1007/s11095-013-1282-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11095-013-1282-2