Abstract
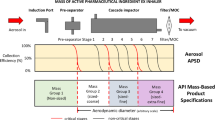
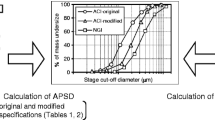
Compendial methods determining dry powder inhaler (DPI)-emitted aerosol aerodynamic particle size distribution (APSD) collect a 4-L air sample containing the aerosol bolus, where the flow, which propagates through the cascade impactor (CI) measurement system from the vacuum source, is used to actuate the inhaler. A previous article described outcomes with two CIs (Andersen eight-stage cascade impactor (ACI) and Next-Generation Pharmaceutical Impactor (NGI)) when the air sample volume was ≤4 L with moderate-resistance DPIs. This article extends that work, examining the hypothesis that DPI flow resistance may be a factor in determining outcomes. APSD measurements were made using the same CI systems with inhalers representing low and high flow resistance extremes (Cyclohaler® and HandiHaler® DPIs, respectively). The ratio of sample volume to internal dead space (normalized volume (V*)) was varied from 0.25 to 1.98 (NGI) and from 0.43 to 3.46 (ACI). Inhaler resistance was a contributing factor to the rate of bolus transfer; the higher resistance DPI completing bolus relocation to the NGI pre-separator via the inlet when V* was as small as 0.25, whereas only ca. 50% of the bolus mass was collected at this condition with the Cyclohaler® DPI. Size fractionation of the bolus from either DPI was completed within the ACI at smaller values of V* than within the NGI. Bolus transfer from the Cyclohaler® capsule and from the HandiHaler® to the ACI system were unaffected by the different flow rise time observed in the two different flow controller systems, and the effects the ACI-based on APSD measurements were marginal.
















Similar content being viewed by others
REFERENCES
European Directorate for the Quality of Medicines and Healthcare (EDQM): European Pharmacopeia 6(8). Chapter 2.9.18. Preparations for inhalations: aerodynamic assessment of fine particles. Strasbourg: Council of Europe; 2010.
US Pharmacopeial Convention. United States Pharmacopeia; USP 33-NF 28; Chapter 601—Physical tests and determinations: aerosols. United States Pharmacopeia, Rockville, MD, USA; 2010.
Copley M, Smurthwaite M, Roberts DL, Mitchell JP. Revised internal volumes to those provided by Mitchell JP and Nagel MW in cascade impactors for the size characterization of aerosols from medical inhalers: their uses and limitations. J Aerosol Med. 2005;18(3):364–6.
Mohammed H, Roberts DL, Copley M, Hammond M, Nichols SC, Mitchell JP. Effect of sampling volume on dry powder inhaler (DPI)-emitted aerosol aerodynamic particle size distributions (APSDs) measured by the Next-Generation Pharmaceutical Impactor (NGI) and the Andersen Eight-Stage Cascade Impactor (ACI). AAPS PharmSciTech. 2012;13(3):875–82.
Copley M. Improving inhaled product testing: methods for obtaining better in vitro-in vivo relationships. Pharm Tech (Europe). 2013;37(2):1–6. Available at: http://www.pharmtech.com/pharmtech/Peer-Reviewed+Research/Improving-Inhaled-Product-Testing-Methods-for-Obta/ArticleStandard/Article/detail/804866 visited June 20, 2013.
De Boer AH, Bolhuis GK, Gjaltema D, Hagedoorn P. Inhalation characteristics and their effects on in vitro drug delivery from dry powder inhalers: part 3: the effect of flow increase rate on the in vitro drug release from the Pulmicort 200 Turbuhaler. Int. J. Pharm. 1997; 153.
Beron KL, Grabek CE, Jung JA, Shelton CM. Flow rate ramp profile effects on the emitted dose from dry powder inhalers. In: Drug delivery to the lungs, 19. Edinburgh, The Aerosol Society; 2008, 61–4.
Van Oort M, Downey B. Cascade impaction of MDIs and DPIs: induction port, inlet cone, and pre-separator lid designs recommended for inclusion in the general test chapter “aerosols” <601> Pharm Forum. 1996;22(2):2204–10.
Marple VA, Roberts DL, Romay FJ, Miller NC, Truman KG, Van Oort M, et al. Next generation pharmaceutical impactor. Part 1: design. J Aerosol Med. 2003;16(3):283–99.
Olsson B, Asking L. Critical aspects of the function of inspiratory flow driven inhalers. J Aerosol Med. 1994;7S1:S43–7.
Donovan MJ, Kim SH, Raman V, Smyth HD. Dry powder inhaler device influence on carrier particle performance. J Pharm Sci. 2012;101(3):1097–107.
Marple VA, Olson BA, Santhanakrishnan K, Mitchell JP, Murray S, Hudson-Curtis B. Next Generation Pharmaceutical Impactor. Part II: calibration. J Aerosol Med. 2003;16:301–24.
Shur J, Lee S, Adams W, Lionberger R, Tibbatts J, Price R. Effect of device design on the in vitro performance and comparability for capsule-based dry powder inhalers. AAPS J. 2012;14(4):667–76.
Nichols SC, Brown DR, Smurthwaite M. New concept for the variable flow rate Andersen cascade impactor and calibration data. J Aerosol Med. 1998;11:133–8.
United States Pharmacopeial Convention. <601 > Inhalation and nasal drug products: aerosols, sprays, and powders—performance quality tests. Pharm Forum 2014;40(2): in press.
Roberts DL. Calibrating cascade impactors with particles—approaches and pitfalls. Inhalation. 2013;7(2):18–23.
United States Pharmacopeial Convention. In process revision: <601 > Inhalation and nasal drug products: aerosols, sprays, and powders—performance quality tests. Pharm. Forum. 2013;39(1) available on-line at: http://www.usppf.com/pf/pub/index.html visited July 9, 2013.
Pedersen S, Hansen OR, Fuglsang G. Influence of inspiratory flow rate upon the effect of a Turbuhaler. Arch Dis Child. 1990;65:308–19.
Broeders MEAC, Molema J, Hop WCJ, Folgering HTM. Inhalation profiles in asthmatics and COPD patients: reproducibility and effects of instruction. J Aerosol Med. 2003;16:131–41.
Mitchell JP, Newman S, Chan H-K. In vitro and in vivo aspects of cascade impactor tests and inhaler performance: a review. AAPS PharmSciTech. 2007;8(4):237–48.
Nadarassan DK, Assi KH, Chrystyn H. Aerodynamic characteristics of a dry powder inhaler at low inhalation flows using a mixing inlet with an Andersen Cascade Impactor. Eur J Pharm Sci. 2010;39:348–54.
Olsson B, Borgström L, Lundbäck H, Svensson M. Validation of a general in vitro approach for prediction of total lung deposition in healthy adults. J. Aerosol Med. Pulmon. Deliv. 2013;26, in press.
ACKNOWLEDGMENTS
The authors wish to acknowledge the support of TEVA-Pharmachemie, Netherlands, and Boehringer-Ingelheim, Germany, for the supply of the DPI products and to other members of the Cascade Impactor Sub-Team of the EPAG for their advice and support during the experimental work and in the internal reviewing of this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mohammed, H., Arp, J., Chambers, F. et al. Investigation of Dry Powder Inhaler (DPI) Resistance and Aerosol Dispersion Timing on Emitted Aerosol Aerodynamic Particle Sizing by Multistage Cascade Impactor when Sampled Volume Is Reduced from Compendial Value of 4 L. AAPS PharmSciTech 15, 1126–1137 (2014). https://doi.org/10.1208/s12249-014-0111-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1208/s12249-014-0111-1