Abstract
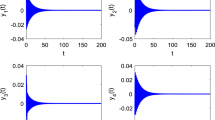
The Hindmarsh–Rose (HR) neuron model has been improved to investigate the complex electrophysiological and various physical phenomena at the level of single cell, for example, time-varying action potential can be induced by the exchange of ion currents and the fluctuation of ions concentration in the cell. When the magnetic flux is considered as a new variable associated to magnetic field, the improved HR neuron model can describe the effects of electromagnetic induction and radiation on membrane potential, where a memristor is used to bridge the membrane potential and the magnetic flux. In this paper, considering the magnetic flux driven, respectively, by the periodic high and low frequency electromagnetic radiation and the Gaussian white noise, the improved HR neuron model is employed to study the modes transition in electrical activities of neuron. The thought-provoking phenomena are detected and discussed by using bifurcation analysis on sampled time series of membrane potential. It is found that the electrical modes of HR neuron model under various parameters have different responses to the periodic high–low frequency electromagnetic radiation and the Gaussian white noise.










Similar content being viewed by others
References
Hodgkin, A.L., Huxley, A.F.: Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J. Physiol. 116, 449–472 (1952)
Hodgkin, A.L., Huxley, A.F.: A quantitative description of membrane current and its application to conduction and excitation in nerve. J. Physiol. 117, 500–544 (1952)
FitzHugh, R.: Impulses and physiological states in theoretical models of nerve membrane. Biophys. J. 1, 445–466 (1961)
Hindmarsh, J.L., Rose, R.M.: A model of the nerve impulse using two first-order differential equations. Nature 296, 162–164 (1982)
Hindmarsh, J.L., Rose, R.M.: A model of neuronal bursting using three coupled first order differential equations. Proc. R. Soc. Lond. B. Biol. Sci. 221, 87–102 (1984)
Burkitt, A.N.: A review of the integrate-and-fire neuron model: I. Homogeneous synaptic input. Biol. Cybern. 95, 1–19 (2006)
Burkitt, A.N.: A review of the integrate-and-fire neuron model: II. Inhomogeneous synaptic input and network properties. Biol. Cybern. 95, 97–122 (2006)
Laing, C.R., Chow, C.C.: A spiking neuron model for binocular rivalry. J. Comput. Neurosci. 12, 39–53 (2002)
Achard, P., Schutter, E.D.: Complex parameter landscape for a complex neuron model. PLoS Comput. Biol. 2, 0794–0804 (2006)
Tsumoto, K., Yoshinaga, T., Aihara, K., Kawakami, H., et al.: Bifurcations in Morris–Lecar neuron model. Neurocomputing 69, 293–316 (2006)
Gu, H.G., et al.: Biological experimental demonstration of bifurcations from bursting to spiking predicted by theoretical models. Nonlinear Dyn. 78, 391–407 (2014)
Gu, H.G., Pan, B.B.: A four-dimensional neuronal model to describe the complex nonlinear dynamics observed in the firing patterns of a sciatic nerve chronic constriction injury model. Nonlinear Dyn. 81, 2107–2126 (2015)
Storace, M., Linaro, D., de Lange, E.: The Hindmarsh–Rose neuron model: bifurcation analysis and piecewise-linear approximations. Chaos 18, 033128 (2008)
Gu, H.G.: Experimental observation of transition from chaotic bursting to chaotic spiking in a neural pacemaker. Chaos 23, 023126 (2013)
Jia, B., Gu, H.G., Xue, L.: A basic bifurcation structure from bursting to spiking of injured nerve fibers in a two-dimensional parameter space. Cogn. Neurodyn. 11, 1–12 (2017)
Zhao, Z.G., Gu, H.G.: Transitions between classes of neuronal excitability and bifurcations induced by autapse. Sci. Rep. 7, 6760 (2017)
Yu, L.C., Chen, Y., Zhang, P.: Frequency and phase synchronization of two coupled neurons with channel noise. Eur. Phys. J. B 59, 249 (2007)
Wang, H.T., Wang, L.F., Yu, L.C., et al.: Response of Morris–Lecar neurons to various stimuli. Phys. Rev. E 83, 021915 (2011)
Chen, Y., Yu, L.C., Qin, S.M.: Detection of subthreshold pulses in neurons with channel noise. Phys. Rev. E 78, 051909 (2008)
Yang, L.J., Liu, W.H., Yi, M., Wang, C., Zhu, Q., Zhan, X., Jia, Y.: Vibrational resonance induced by transition of phase-locking modes in excitable system. Phys. Rev. E 86, 016209 (2012)
Bao, B.C., Liu, Z., Xu, J.P.: Steady periodic memristor oscillator with transient chaotic behaviours. Electron. Lett. 46, 237–238 (2010)
Muthuswamy, B.: Implementing memristor based chaotic circuits. Int. J. Bifurc. Chaos 20, 1335–1350 (2010)
Pinto, R.D., Varona, P., Volkovskii, A.R., et al.: Synchronous behavior of two coupled electronic neurons. Phys. Rev. E 62, 2644 (2000)
Selverston, A., Rabinovich, M., Abarbanel, H.D., et al.: Reliable circuits for irregular neurons: a dynamical approach to understanding central pattern generators. J. Physiol. 94, 357–374 (2000)
Wu, X.Y., Ma, J., Yuan, L.H., et al.: Simulating electric activities of neurons by using PSPICE. Nonlinear Dyn. 75, 113–126 (2014)
Herrmann, C.S., Klaus, A.: Autapse turns neuron into oscillator. Int. J. Bifurcat. Chaos 14, 623–633 (2004)
Li, Y.Y., Schmid, G., Hänggi, P., et al.: Spontaneous spiking in an autaptic Hodgkin–Huxley set up. Phys. Rev. E 82, 061907 (2012)
Ren, G.D., Wu, G., Ma, J., et al.: Simulation of electric activity of neuron by setting up a reliable neuronal circuit driven by electric autapse. Acta Phys. Sin. 64, 058702 (2015)
Song, X.L., Wang, C.N., Ma, J., et al.: Transition of electric activity of neurons induced by chemical and electric autapses. Sci. China Technol. Sci. 58, 1007–1014 (2015)
Xu, Y., Ying, H., Jia, Y., et al.: Autaptic regulation of electrical activities in neuron under electromagnetic. Sci. Rep. 7, 43452 (2017)
Guo, S.L., Tang, J., Ma, J., et al.: Autaptic modulation of electrical activity in a network of neuron-coupled astrocyte. Complexity 2017, 1–13 (2017). https://doi.org/10.1155/2017/4631602
Wang, C.N., Guo, S.L., Xu, Y., et al.: Formation of autapse connected to neuron and its biological function. Complexity 2017, 1–9 (2017). https://doi.org/10.1155/2017/5436737
Ma, J., Song, X.L., Tang, J., et al.: Wave emitting and propagation induced by autapse in a forward feedback neuronal network. Neurocomputing 167, 378–389 (2015)
Ma, J., Tang, J.: A review for dynamics of collective behaviors of network of neurons. Sci. China Technol. Sci. 58, 2038–2045 (2015)
Harris, J.J., Jolivet, R., Attwell, D.: Synaptic energy use and supply. Neuron 75, 762–77 (2012)
Wang, R.B., Zhang, Z.K., Chen, G.R.: Energy coding and energy functions for local activities of the brain. Neurocomputing 73, 139–150 (2009)
Torrealdea, F.J., Sarasola, C., d’Anjou, A., Moujahid, A., et al.: Energy efficiency of information transmission by electrically coupled neurons. Biosystems 97, 60–71 (2009)
Wang, Z.Y., Wang, R.B., Fang, R.Y.: Energy coding in neural network with inhibitory neurons. Cogn. Neurodyn. 9, 129–144 (2015)
Zheng, H.W., Wang, R.B., Qu, J.Y.: Effect of different glucose supply conditions on neuronal energy metabolism. Cogn. Neurodyn. 10, 563–571 (2016)
Wang, R.B., Zhang, Z.K.: Energy coding in biological neural networks. Cogn. Neurodyn. 1, 203–212 (2007)
Wang, R.B., Zhu, Y.T.: Can the activities of the large scale cortical network be expressed by neural energy? A brief review. Cogn. Neurodyn. 10, 1–5 (2016)
Wang, Y., Wang, C.N., Ren, G.D., et al.: Energy dependence on modes of electric activities of neuron driven by multi-channel signals. Nonlinear Dyn. 89, 1967–1987 (2017)
Yu, L.C., Liu, L.W.: Optimal size of stochastic Hodgkin–Huxley neuronal systems for maximal energy efficiency in coding pulse signals. Phys. Rev. E 89, 032725 (2014)
Yu, L.C., Zhang, C., Liu, L.W., et al.: Energy-efficient population coding constrains network size of a neuronal array system. Sci. Rep. 6, 19369 (2016)
Yu, L.C., Yu, Y.: Energy-efficient neural information processing in individual neurons and neuronal networks. J. Neurosci. Res. 95, 2253–2266 (2017)
Li, J.J., Liu, S.B., Liu, W.M., Yu, Y.G., Wu, Y.: Suppression of firing activities in neuron and neurons of network induced by electromagnetic radiation. Nonlinear Dyn. 83, 801–810 (2016)
Wang, H.T., Chen, Y.: Spatiotemporal activities of neural network exposed to external electric fields. Nonlinear Dyn. 85, 881–891 (2016)
Lv, M., Wang, C.N., Ren, G.D., Ma, J.: Model of electrical activity in a neuron under magnetic flow effect. Nonlinear Dyn. 85, 1479–1490 (2016)
Lv, M., Ma, J.: Multiple modes of electrical activities in a new neuron model under electromagnetic radiation. Neurocomputing 205, 375–381 (2016)
Li, Q.D., Zeng, H.Z., Li, J.: Hyperchaos in a 4D memristive circuit with infinitely many stable equilibria. Nonlinear Dyn. 79, 2295–2308 (2015)
Gu, H.G., Pan, B.B.: Identification of neural firing patterns, frequency and temporal coding mechanisms in individual aortic baroreceptors. Front. Comput. Neurosci. 9, 108 (2015)
Jia, B., Gu, H.G.: Dynamics and physiological roles of stochastic neural firing patterns near bifurcation points. Int. J. Bifurcat. Chaos 27, 1750113 (2017)
Wu, F.Q., Wang, C.N., Jin, W.Y., et al.: Dynamical responses in a new neuron model subjected to electromagnetic induction and phase noise. Physica A 469, 81–88 (2017)
Wu, J., Xu, Y., Ma, J.: Lévy noise improves the electrical activity in a neuron under electromagnetic radiation. PLoS One 12, e0174330 (2017)
Wang, Y., Ma, J., Xu, Y., et al.: The electrical activity of neurons subject to electromagnetic induction and Gaussian white noise. Int. J. Bifurcat. Chaos 27, 1750030 (2017)
Lu, L.L., Jia, Y., Liu, W.H., Yang, L.J.: Mixed stimulus-induced model selection in neural activity driven by high and low frequency current under electromagnetic radiation. Complexity 2017, 1–11 (2017). https://doi.org/10.1155/2017/7628537
Wang, L.F., Qiu, K., Jia, Y.: Effects of time delays in a mathematical bone model. Chin. Phys. B 26, 030503 (2017)
Qiu, K., Gao, K.F., et al.: A kinetic model of multiple phenotypic states for breast cancer cells. Sci. Rep. 7, 9890 (2017)
Qiu, K., Wang, L.F., Shen, J., et al.: A van der Waals-like transition between normal and cancerous phases in cell populations dynamics of colorectal cancer. Sci. Rep. 6, 36620 (2016)
Pei, Q.M., Zhan, X., Yang, L.J., et al.: Fluctuation and noise propagation in phenotypic transition cascades of clonal populations. Phys. Rev. E 92, 012721 (2015)
Acknowledgements
The authors gratefully acknowledge Prof. Jun Ma from Lanzhou University of Technology for the constructive suggestions. This work was supported by the National Natural Science Foundation of China under 11775091 and 11474117.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ge, M., Jia, Y., Xu, Y. et al. Mode transition in electrical activities of neuron driven by high and low frequency stimulus in the presence of electromagnetic induction and radiation. Nonlinear Dyn 91, 515–523 (2018). https://doi.org/10.1007/s11071-017-3886-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11071-017-3886-2