Abstract
Astrocytes play a dual role in the brain. On the one hand, they are active signaling partners of neurons and can for instance control synaptic transmission and its plasticity. On the other hand, they fulfill various homeostatic functions such as clearance of glutamate and K+ released from neurons. The latter is for instance important for limiting neuronal excitability. Therefore, an impairment or failure of glutamate and K+ clearance will lead to increased neuronal excitability, which could trigger or aggravate brain diseases such as epilepsy, in which neuronal hyperexcitability plays a role. Experimental data indicate that astrocytes could have such a causal role in epilepsy, but the role of astrocytes as initiators of epilepsy and the relevant mechanisms are under debate. In this overview, we will discuss the potential mechanisms with focus on K+ clearance, glutamate uptake and homoeostasis and related mechanisms, and the evidence for their causative role in epilepsy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytes are a subtype of glial cell in the brain. They play many physiological roles that range from neurotransmitter uptake to the modulation of synaptic transmission and plasticity [1]. Profound changes of astrocyte properties and function in brain diseases such as epilepsy are a common and widespread finding. Such alterations of astrocytes can be found on the level of protein expression, morphology, and operation of signaling cascades, which can contribute to the phenotype and symptoms of the disease and to disease progression although many disease-specific mechanisms remain to be fully understood [2]. A key question is often whether astrocyte changes in disease are the origin of the disease or a consequence of it. For epilepsy, which is a heterogenous group of neurological disorder characterized by recurrent epileptic seizures, this is an intensely discussed question. In the following, we will therefore discuss by which mechanisms astrocytes could play a role as initiators of epilepsy and what the experimental evidence for such a causative role of astrocytes is. We will limit our discussion to temporal lobe epilepsy (TLE), a common and often drug-resistant form of epilepsy.
One strategy for revealing if astrocyte mechanisms can initiate and promote epileptic activity, is to identify astrocytic changes in epileptic tissue and then test if disruption of such candidate mechanisms affects epilepsy and if reproducing a specific astrocytic change is sufficient to induce epilepsy. Relevant information about astrocytic changes has been gained from epileptogenic brain tissue specimens surgically resected from patients with drug resistant TLE showing hippocampal sclerosis (HS). Both TLE and HS are strongly associated with an initial precipitating event such as febrile seizures, trauma, hypoxia, or brain infections [3]. In humans, chronic TLE usually does not develop immediately after such an event but following a seizure-free period that can last many years and is referred to as the latent period [4]. The latter is of particular interest for epilepsy research as during this period, pathophysiological changes occur that eventually culminate in chronic epilepsy. Since the latent period cannot be studied in human tissue, animal models are required that reproduce this typical pattern of epileptogenesis. This is also important because an astrocytic change supposedly causing the development of epilepsy needs to occur before the onset of epilepsy and epileptic neuronal activity. Also, the availability of live human tissue is limited and experimental work in live human tissue with HS is challenging. Therefore, several post-status epilepticus (SE) models have been investigated in which systemic or local administration of chemoconvulsants or electrical stimulation triggers SE and the development of chronic epilepsy after a latent period of days to weeks [5,6,7,8]. Accumulating evidence from these models suggests that astrocytes become dysfunctional immediately after the initial precipitating event or during the latent period. Accordingly, these changes could be causative in epileptogenesis. It is important to note here that the properties of astrocytes in acute slices from human cortical specimens (resected to gain access to the epileptogenic area) or non-sclerotic hippocampal slices from patients with ‘lesion-associated’ TLE that lack significant histopathological hippocampal alterations were remarkably similar to those from the corresponding brain areas of healthy rodents [9].
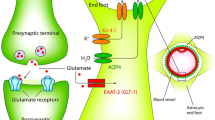
Schematic illustration of astrocyte changes in the healthy (left) vs. epileptic (right) brain. (1) In epilepsy, astrocytes lose their ability to form functional GJ-coupled networks, resulting in increased extracellular K+ concentrations. (2) The density of AQP4 channels along the perivascular membrane domain of astrocytes is reduced in the epileptic brain, leading to a dysregulation of water transport and a concomitant decrease in the extracellular space volume as well as an increase of the extracellular fluid osmolarity. Additionally, downregulation of astrocytic Kir4.1 channels contributes to impaired K+ buffering in both human and experimental epilepsy. (3) Seizure-induced disruption of the BBB results in albumin extravasation and subsequent TGF-βR-mediated astrocyte dysfunction. (4) Perturbations in astrocyte-dependent glutamate homeostasis including impaired glutamate uptake, reduced GS efficiency and aberrant glutamate release by astrocytes contribute to increased extracellular glutamate levels. (5) Overexpression of ADK in epileptic tissue decreases the ambient adenosine concentration and could amplify neuronal excitability
Involvement of Kir4.1 Channels
There are several consistent findings regarding astrocyte changes in epileptic tissue (Fig. 1). For instance, the ability of astrocytes in human HS to fulfill one of its essential functions, the spatial buffering of K+ [10], has already been investigated more than 20 years ago [11,12,13]. These authors showed that specific inhibition of glial inwardly rectifying K+ (Kir4.1) channels, which mediate the passive uptake of K+ by astrocytes, substantially augmented stimulus induced or iontophoretically applied elevations in extracellular K+ in non-sclerotic human hippocampal slices but had no effect in human HS. These results provided first evidence for the disturbance of K+ buffering in HS. Further evidence came from comparative patch-clamp studies showing a significant reduction of astrocytic Kir currents in HS [14,15,16]. In line with this, significantly reduced Kir4.1 protein levels were detected by immunohistochemical and western blot analysis in patients with HS compared to non-sclerotic and autopsy controls [17,18,19]. Importantly, genetic linkage studies have indicated an association between missense variations in the human Kir4.1 gene (KCNJ10) and seizure susceptibility in TLE [20, 21]. For these reasons, impaired K+ buffering via Kir4.1 is a candidate mechanism in epileptogenesis.
Insights into the importance of Kir4.1 channels for K+ buffering and regulation of neuronal excitability particularly emerged from Kir4.1 knockout (KO) mice, which exhibit impaired K+ clearance and an epileptic phenotype [22, 23]. However, whether changes in Kir4.1 represent a causal event in epileptogenesis is still unclear because data on its expression during the latent period are inconsistent among experimental TLE models. For instance, in an albumin model downregulation of Kir4.1 transcripts and reduced K+ clearance were detected long before the onset of epileptic activity [24], while no changes in Kir4.1-mediated K+ currents could be found in hippocampal astrocytes during the latent period of experimental TLE induced by systemic injection of kainate [25].
Uncoupling of Astrocytes
In addition to Kir4.1, spatial K+ buffering may also depend on the interconnection of astrocytes through gap junction channels. To our knowledge, characterization of functional gap junction coupling between human hippocampal astrocytes has been performed in only one study [6]. Here, tracer diffusion assays demonstrated a complete loss of astrocytic coupling in human HS, whereas in non-sclerotic control tissue the extent of coupling was similar to that observed in rodents [6, 9]. Interestingly, it was shown that this loss of coupling is not due to decreased expression of gap junction proteins (connexins, Cx), but rather the result of altered subcellular localization and phosphorylation of Cx43 [26]. The pathological consequences of impaired gap junctional coupling can be seen in patients with oculodentodigital dysplasia (ODDD), a rare genetic disease caused by mutations in the gene encoding Cx43. These patients present with epileptic seizures in addition to other neurological symptoms [27, 28]. These observations imply that perturbed gap junction coupling is an additional candidate mechanism in epileptogenesis. This is also suggested by a recent study revealing that gap junction coupling plays a selective role in buffering high local K+ increases [29], which are typical for epilepsy.
Experimental support for the importance of astrocytic gap junction channels in K+ clearance and neuronal hyperexcitability has been gained in hippocampal slices from transgenic mice with coupling-deficient astrocytes [30, 31] and with pharmacological disruption of gap junction communication in situ and in vivo [29, 32, 33]. Analyses of transgenic mice with coupling-deficient astrocytes further revealed that not only K+ buffering but also glutamate clearance is impaired when astrocyte Cxs are absent [30]. Consistently, acute slices from these mice displayed spontaneous epileptiform events and substantially increased seizure and interictal spike activity during the chronic phase of experimentally induced TLE [31, 34]. However, neither constitutive nor inducible astrocytic Cx KO mice showed spontaneous behavioral seizures or abnormal EEG activity in vivo [35, 36]. It has to be considered that in the latter studies, mice with complete or significant deletion of Cx43 and Cx30 were used, while loss of coupling in HS was not accompanied by any reduction in Cx proteins [26]. Complete loss of gap junction coupling as well as the subcellular reorganization and altered phosphorylation of Cx43 characterizing human HS could be reproduced in the intracortical kainate injection mouse model of TLE-HS [6, 26]. Intriguingly, in this model, loss of astrocyte coupling and the resulting impairment in K+ clearance temporally precede neuronal death and onset of spontaneous seizure activity, pointing to a causal role of astrocyte dysfunction in the initiation of TLE [6].
The signaling pathway underlying loss of astrocyte coupling in TLE remains unknown. One potential mechanism can be inferred from the observation that epilepsy is associated with a breakdown of the blood-brain barrier (BBB), which results in extravasation of serum proteins, including albumin, into the brain parenchyma [37, 38]. Extravasated albumin is endocytosed by astrocytes [39,40,41] via binding to transforming growth factor beta (TGFß) receptors [42,43,44]. Experimental albumin infusions impair interastrocytic gap junction coupling and extracellular K+ buffering, probably due to TGFß signaling-dependent transcriptional downregulation of astrocytic Cxs and Kir4.1 [24, 39, 43, 44]. Similarly, albumin-induced TGFß signaling has been shown to induce epileptiform activity in situ and in vivo [42, 43, 45]. In animal models of TLE, BBB opening and albumin extravasation occur within hours of the precipitating insult [46,47,48]. However, data examining consequences of albumin induced TGFß signaling in astrocytes in experimental TLE are limited. In a recent study performed in our lab, astrocytic albumin uptake 4 and 24 h after kainate-induced SE was negligible. Early short-term TGFβR1 kinase inhibition did not prevent seizure-induced gap junction uncoupling in astrocytes and exerted only minor effects on acute and chronic epileptiform activity [48]. In contrast, long-term treatment with the angiotensin II type 1 receptor inhibitor losartan, which also inhibits TGFβ signaling [42], reduces seizure frequency and attenuates hippocampal neurodegeneration and behavioral abnormalities in kainate-induced epilepsy in rats [49]. Together, these findings indicate that sustained inhibition of albumin-induced TGFß signaling could be necessary to affect epileptogenesis. This hypothesis is also supported by the observation that albumin extravasation is a phenomenon that persists until the chronic phase of experimental and human TLE [26]. Two other recent studies performed in rats showed that inhibition of TGFß1 signaling attenuates kainate-induced seizures and astrogliosis [50, 51]. Unfortunately, the two latter studies did not determine whether neuronal or astrocyte TGFß signaling was affected, and the outcome of TGFß inhibition on development of chronic seizure activity was also not addressed. Thus, further investigations are needed to decide whether albumin induced TGFß signaling is causally involved in astrocyte dysfunction and epileptogenesis.
Perturbed Glutamate and Adenosine signaling
Glutamate transport and homoeostasis are also implicated in epileptogenesis and controlled by astrocytes. For instance, astrocytes are believed to mediate most of the uptake of glutamate released from neurons [52, 53], which is a central mechanism ensuring physiological excitatory neurotransmission and protection from excitotoxicity [54, 55]. Astrocytic glutamate uptake is accomplished by the glia-specific transporter EAAT1 (GLAST) and by EAAT2 (GLT-1). Glutamate is thought to be then converted into glutamine by the enzyme glutamine synthetase (GS), which is then shuttled back to neurons for the resynthesis of glutamate [53, 56]. Extracellular glutamate levels are elevated in the human hippocampus of TLE patients, especially before and during seizure activity [57, 58]. There are at least three ways this can be explained by an astrocyte dysfunction. First, glutamate uptake by astrocytes or its metabolism could be impaired [59]. Second, astrocytic regulation of neuronal excitability could fail leading to increases in neuronal glutamate release. Third, astrocytes could release the additional glutamate themselves.
Regarding astrocytic glutamate uptake, immunostaining studies reported downregulation of the protein levels of both glutamate transporters in human HS [60,61,62], although other investigators found no changes [63, 64]. The glutamate sensitivity of human glia cells has been assessed in one study by rapid application of glutamate to outside-out patches excised from glia cells in acute hippocampal slices from TLE-HS patients [6]. The results of this study suggest loss of functional transporters and aberrant expression of AMPA receptors in astrocytes, although the identity of the glial cells residing in human HS remained unclear.
It is important to note that glutamate transport is regulated on many levels, which could be important for epileptogenesis. For instance, the mobility of the glutamate transporter GLT-1 on the astrocyte surface has been shown to be activity and location-dependent [65]. Also, glutamate transport is rapidly modulated by burst-like neuronal activity [66], which adds another layer of complexity to glutamate uptake. Importantly, the efficacy of glutamate uptake also depends on the relative position of astrocytic perisynaptic processes and active excitatory synapses. The deletion of the gap junction protein Cx30 for instance resulted in the invasion of the synaptic cleft by astrocytic processes, increased glutamate uptake and decreased excitatory synaptic transmission [67], which links astrocyte gap junction coupling to glutamate homoeostasis. Furthermore, we have recently demonstrated that the relative astrocytic coverage of excitatory synapses correlates with the local efficacy of glutamate uptake and shielding of synapses from invading glutamate from other sources [68], which indicates that epilepsy-associated morphology changes could have a profound effect on glutamate clearance and spread in the tissue. Similarly, perisynaptic astrocytic processes withdraw after the induction of long-term potentiation of synaptic transmission using a high-frequency stimulus, which also increased glutamate spread in the tissue and promoted synaptic crosstalk [69]. This raises the question if transiently increased neuronal activity induces a similar rapid remodeling of astrocytes and whether that promotes or triggers epileptogenesis. The possibility of such a mechanism is also suggested by the following observations. On the one hand, astrocyte morphology is controlled by small GTPases of the Rho family and downstream kinases such as the Rho-associated protein kinase (ROCK) [70, 71]. On the other hand, pharmacological inhibition of ROCK decreased the severity of seizures in the PTZ kindling model [72] and reduced neurodegeneration in a kainic acid epilepsy model [73].
Interesting insights were also obtained about the role of GS in human HS. Here, a pronounced reduction of the enzyme and its functional activity was described [64, 74]. Direct evidence for the involvement of GS deficiency in epilepsy is given by the fact that patients with congenital, homozygous mutations in the GS gene display severe brain malformations and epileptic seizures [75, 76]. Interestingly, experimental induction of reactive gliosis was shown to reduce the expression of GS in the hippocampus, to reduce GABAergic synaptic inhibition but not excitatory synaptic transmission, and to render the hippocampal circuit hyperexcitable [77].
As pointed out above, a failure of astrocytes to limit excitability of neurons could lead to the observed increase of glutamate levels in TLE and HS. Impairment or failure of astrocyte K+ buffering is one potential mechanisms (see previous section). Another relevant one is the astrocytic control of the excitability of neuronal networks through extracellular concentrations of adenosine via the enzyme adenosine kinase (ADK). As adenosine exerts powerful anticonvulsive and neuroprotective effects by acting on pre- or postsynaptic A1 receptors, alterations in ADK expression are thought to play a crucial role in epilepsy [78, 79]. Baseline adenosine levels in microdialysis samples from epileptic patients are relatively low, while they rapidly rise during seizures, a process hypothesized to mediate seizure termination and postictal suppression [80]. Using immunocytochemistry and Western blot analysis, Aronica and colleagues [81] demonstrated marked overexpression of astrocytic and total ADK protein levels in the sclerotic hippocampi of TLE patients, a phenomenon they considered a common pathologic hallmark of medically intractable chronic epilepsy.
Increased ADK expression and impaired adenosine-mediated inhibition have also been implicated in experimental TLE [78, 82]. For example, knock-down of ADK using ADK-targeting microRNA attenuated kainate-induced acute seizures [83], and pharmacological inhibition of ADK during the chronic phase of KA-induced epilepsy ameliorates seizures [84]. Moreover, overexpression of ADK in the brain induces hyperexcitability and seizures [85, 86], while adenosine augmentation therapies possess seizure suppressing and anti-epileptogenic effects [87,88,89,90]. Interestingly, ADK expression depends on the stage of epilepsy, with decreased expression immediately following intrahippocampal kainate injection, but increased expression during the latent or chronic periods (≥ 3 d) of epilepsy [84, 91]. Accordingly, transient administration of an ADK inhibitor during a period of elevated ADK expression in the latent period reduced seizure activity and granule cell dispersion at later stages of the disease [91]. Together these findings support an important role of adenosine in epilepsy and indicate the potential of ADK-targeting and adenosine-enhancing therapies for the treatment of the disease. It should be noted however that ADK is primarily expressed by astrocytes and other glial cells in rodents [92, 93], whereas its expression is more homogeneous across cell types according to human sequencing data [94].
Another possible explanation for the increased glutamate concentrations in epileptic tissue could be excessive astrocytic release of the neurotransmitter. Indeed, astrocytes are believed to not only detect and react to neuronal activity, but also to respond and actively regulate neuronal excitability and synaptic transmission through Ca2+-dependent release of neuroactive substances (so-called gliotransmitters like glutamate, ATP, and D-serine) [95, 96]. Such bidirectional signaling between astrocytes and neurons has also been demonstrated in human brain tissue from drug resistant TLE patients, but it remained unclear whether this represented a pathological or a physiological phenomenon, as control tissue was not analyzed [97]. Up-regulation of astrocytic metabotropic glutamate receptors (mGluRs), which are also involved in neuron-glia interactions, has been demonstrated in human TLE [98,99,100,101] and may indicate altered gliotransmission. However, direct evidence for an involvement of astrocytic glutamate release in triggering epileptic activity is still missing to our knowledge.
Dysregulation of Water Flux
Extracellular K+ and neurotransmitter concentrations and dynamics are not only dependent on astrocyte uptake and clearance mechanisms, but also on the volume of the extracellular space (ECS), which is regulated by a family of membrane channels termed aquaporins (AQPs). The predominant isoform of aquaporins in adult brain, AQP4, is expressed in close association with Kir4.1 channels in astrocytic perivascular endfeet and perisynaptic processes.
In sclerotic hippocampi from TLE patients the overall expression of AQP4 protein is increased [102, 103], but the density of the water channels along the perivascular membrane domain of astrocytes is reduced. This perivascular AQP4 loss resulted from decreased perivascular expression of the anchoring protein dystrophin and was postulated to perturb the flux of water and K+ through astrocytes and consequently increase the occurrence of seizures [102, 103]. As in the case of the Kir4.1 gene, KCNJ10, genetic studies revealed several SNPs in the human AQP4 genes that were associated with TLE [21].
In animal models of epilepsy, AQP4 dysregulation occurs during the early phase of epileptogenesis, which suggest that it is a potential driver of epileptogenesis [104,105,106,107]. Moreover, studies employing AQP4 KO mice provided evidence for a causal involvement of AQP4 channels in the disease. For example, AQP4 KO mice are characterized by increased spontaneous recurrent seizures and neuronal loss following kainate-induced SE [107]. Similarly, AQP4 KO mice display increased seizure duration and altered EEG power spectra in experimental posttraumatic epilepsy [108]. Mechanistically, lack of AQP4 may contribute to the pathophysiology of epilepsy due to the role of water channels in regulating ECS volume and osmolarity, as well as its involvement in K+ homeostasis [82, 109]. Notably, constitutive deletion of AQP4 is accompanied by enhanced astrocyte gap junction coupling and K+ buffering [110], which complicates the interpretation of data from AQP4 deficient mice. Nonetheless, these studies collectively indicate that AQP4 can play an important role in the initiation of epilepsy.
Conclusions and Future Directions
There is substantial experimental evidence for several astrocytic candidate mechanisms that could initiate the development of TLE. For the sake of conciseness, we have focused on astrocytic K+ clearance and glutamate homeostasis and a few relevant aspects, such as K+ channels, astrocytic gap junction coupling, glutamate uptake and metabolism, water transport and adenosine signaling (Fig. 1). This list is not exhaustive, but these examples highlight candidate mechanisms supported by particularly strong evidence obtained in epilepsy models of TLE and human TLE.
As discussed above, for a perturbation of astrocyte function (e.g. change of protein expression, function, signaling) to be causal in the development of TLE it needs to meet several requirements. For instance, it must occur before the onset of epilepsy. Also, preventing that astrocytic change should prevent the development of TLE too, for instance in an animal model. Ideally, reproducing that astrocyte change in isolation would also lead to the development of TLE. Establishing causality to this extent remains a challenge. Astrocytic gap junction uncoupling is a good example (see above for details). The uncoupling occurs before the onset of chronic epilepsy. However, complete inhibition of coupling in genetically modified mice does not directly lead to chronic epilepsy, which suggest that more than one factor is involved. Identifying those additional factors remains a challenge. Also, preventing uncoupling experimentally in an epilepsy model is difficult because it requires precise knowledge of how uncoupling was triggered mechanistically. A follow-up question is then if what was learnt about additional factors and mechanisms leading to the development of chronic seizure in a TLE model using, for instance, chemoconvulsants can be transferred to human TLE where the triggers and how they lead to TLE is incompletely understood. Naturally, these considerations apply to any astrocytic candidate mechanism for the initiation of TLE. Using mice where epilepsy-relevant glial candidate genes are selective and inducibly deleted or manipulated might be of great help in answering some of the open questions.
Abbreviations
- ADK:
-
adenosine kinase
- AMP:
-
adenosine monophosphate
- AQP4:
-
aquaporin 4
- BBB:
-
blood-brain barrier
- ER:
-
endoplasmic reticulum
- GJC:
-
gap junction channel
- GS:
-
glutamine synthetase
- IP3 :
-
inositol triphosphate
- Kir4.13 :
-
inwardly-rectifying K+ channel 4.1
- mGluR3 :
-
metabotropic glutamate receptor
- PAP3 :
-
perisynaptic astrocyte process
- TGF-ßR3 :
-
transforming growth-factor ß receptor
References
Verkhratsky A, Nedergaard M (2018) Physiology of Astroglia. Physiol Rev 98:239–389. https://doi.org/10.1152/physrev.00042.2016
Escartin C, Galea E, Lakatos A et al (2021) Reactive astrocyte nomenclature, definitions, and future directions. Nat Neurosci. https://doi.org/10.1038/s41593-020-00783-4
Mathern GW, Babb TL, Vickrey BG et al (1995) The clinical-pathogenic mechanisms of hippocampal neuron loss and surgical outcomes in temporal lobe epilepsy. Brain 118:105–118. https://doi.org/10.1093/brain/118.1.105
Clossen BL, Reddy DS (2017) Novel therapeutic approaches for disease-modification of epileptogenesis for curing epilepsy. Biochim Biophys Acta BBA - Mol Basis Dis 1863:1519–1538. https://doi.org/10.1016/j.bbadis.2017.02.003
Löscher W, Schmidt D (2011) Modern antiepileptic drug development has failed to deliver: ways out of the current dilemma. Epilepsia 52:657–678. https://doi.org/10.1111/j.1528-1167.2011.03024.x
Bedner P, Dupper A, Hüttmann K et al (2015) Astrocyte uncoupling as a cause of human temporal lobe epilepsy. Brain 138:1208–1222. https://doi.org/10.1093/brain/awv067
Rusina E, Bernard C, Williamson A (2021) The Kainic Acid Models of Temporal Lobe Epilepsy. https://doi.org/10.1523/ENEURO.0337-20.2021. eNeuro 8:
Jefferys J, Steinhäuser C, Bedner P (2016) Chemically-induced TLE models: Topical application. J Neurosci Methods 260:53–61. https://doi.org/10.1016/j.jneumeth.2015.04.011
Bedner P, Jabs R, Steinhäuser C (2020) Properties of human astrocytes and NG2 glia. Glia 68:756–767. https://doi.org/10.1002/glia.23725
Orkand RK (1986) Introductory Remarks: Glial-Interstitial Fluid Exchange. Ann N Y Acad Sci 481:269–272. https://doi.org/10.1111/j.1749-6632.1986.tb27157.x
Heinemann U, Gabriel S, Jauch R et al (2000) Alterations of Glial Cell Function in Temporal Lobe Epilepsy. Epilepsia 41:S185–S189. https://doi.org/10.1111/j.1528-1157.2000.tb01579.x
Jauch R, Windmüller O, Lehmann T-N et al (2002) Effects of barium, furosemide, ouabaine and 4,4′-diisothiocyanatostilbene-2,2′-disulfonic acid (DIDS) on ionophoretically-induced changes in extracellular potassium concentration in hippocampal slices from rats and from patients with epilepsy. Brain Res 925:18–27. https://doi.org/10.1016/S0006-8993(01)03254-1
Kivi A, Lehmann T-N, Kovács R et al (2000) Effects of barium on stimulus-induced rises of [K+]o in human epileptic non-sclerotic and sclerotic hippocampal area CA1. Eur J Neurosci 12:2039–2048. https://doi.org/10.1046/j.1460-9568.2000.00103.x
Hinterkeuser S, Schröder W, Hager G et al (2000) Astrocytes in the hippocampus of patients with temporal lobe epilepsy display changes in potassium conductances. Eur J Neurosci 12:2087–2096. https://doi.org/10.1046/j.1460-9568.2000.00104.x
Schröder W, Hinterkeuser S, Seifert G et al (2000) Functional and molecular properties of human astrocytes in acute hippocampal slices obtained from patients with temporal lobe epilepsy. Epilepsia 41(Suppl 6):S181–184
Bordey A, Sontheimer H (1998) Electrophysiological Properties of Human Astrocytic Tumor Cells In Situ: Enigma of Spiking Glial Cells. J Neurophysiol 79:2782–2793. https://doi.org/10.1152/jn.1998.79.5.2782
Heuser K, Eid T, Lauritzen F et al (2012) Loss of Perivascular Kir4.1 Potassium Channels in the Sclerotic Hippocampus of Patients With Mesial Temporal Lobe Epilepsy. J Neuropathol Exp Neurol 71:814–825. https://doi.org/10.1097/NEN.0b013e318267b5af
Kitaura H, Shirozu H, Masuda H et al (2018) Pathophysiological Characteristics Associated With Epileptogenesis in Human Hippocampal Sclerosis. EBioMedicine 29:38–46. https://doi.org/10.1016/j.ebiom.2018.02.013
Das A, Wallace GC, Holmes C et al (2012) Hippocampal tissue of patients with refractory temporal lobe epilepsy is associated with astrocyte activation, inflammation, and altered expression of channels and receptors. Neuroscience 220:237–246. https://doi.org/10.1016/j.neuroscience.2012.06.002
Buono RJ, Lohoff FW, Sander T et al (2004) Association between variation in the human KCNJ10 potassium ion channel gene and seizure susceptibility. Epilepsy Res 58:175–183. https://doi.org/10.1016/j.eplepsyres.2004.02.003
Heuser K, Nagelhus EA, Taubøll E et al (2010) Variants of the genes encoding AQP4 and Kir4.1 are associated with subgroups of patients with temporal lobe epilepsy. Epilepsy Res 88:55–64. https://doi.org/10.1016/j.eplepsyres.2009.09.023
Chever O, Djukic B, McCarthy KD, Amzica F (2010) Implication of Kir4.1 Channel in Excess Potassium Clearance: An In Vivo Study on Anesthetized Glial-Conditional Kir4.1 Knock-Out Mice. J Neurosci 30:15769–15777. https://doi.org/10.1523/JNEUROSCI.2078-10.2010
Haj-Yasein NN, Jensen V, Vindedal GF et al (2011) Evidence that compromised K + spatial buffering contributes to the epileptogenic effect of mutations in the human kir4.1 gene (KCNJ10). Glia 59:1635–1642. https://doi.org/10.1002/glia.21205
David Y, Cacheaux LP, Ivens S et al (2009) Astrocytic Dysfunction in Epileptogenesis: Consequence of Altered Potassium and Glutamate Homeostasis? J Neurosci 29:10588–10599. https://doi.org/10.1523/JNEUROSCI.2323-09.2009
Takahashi DK, Vargas JR, Wilcox KS (2010) Increased coupling and altered glutamate transport currents in astrocytes following kainic-acid-induced status epilepticus. Neurobiol Dis 40:573–585. https://doi.org/10.1016/j.nbd.2010.07.018
Deshpande T, Li T, Herde MK et al (2017) Subcellular reorganization and altered phosphorylation of the astrocytic gap junction protein connexin43 in human and experimental temporal lobe epilepsy. Glia 65:1809–1820. https://doi.org/10.1002/glia.23196
Loddenkemper T, Grote K, Evers S et al (2002) Neurological manifestations of the oculodentodigital dysplasia syndrome. J Neurol 249:584–595. https://doi.org/10.1007/s004150200068
Walrave L, Vinken M, Leybaert L, Smolders I (2020) Astrocytic Connexin43 Channels as Candidate Targets in Epilepsy Treatment. Biomolecules 10:1578. https://doi.org/10.3390/biom10111578
Breithausen B, Kautzmann S, Boehlen A et al (2020) Limited contribution of astroglial gap junction coupling to buffering of extracellular K + in CA1 stratum radiatum. Glia 68:918–931. https://doi.org/10.1002/glia.23751
Pannasch U, Vargová L, Reingruber J et al (2011) Astroglial networks scale synaptic activity and plasticity. Proc Natl Acad Sci U S A 108:8467–8472. https://doi.org/10.1073/pnas.1016650108
Wallraff A, Köhling R, Heinemann U et al (2006) The Impact of Astrocytic Gap Junctional Coupling on Potassium Buffering in the Hippocampus. J Neurosci 26:5438–5447. https://doi.org/10.1523/JNEUROSCI.0037-06.2006
Bazzigaluppi P, Weisspapir I, Stefanovic B et al (2017) Astrocytic gap junction blockade markedly increases extracellular potassium without causing seizures in the mouse neocortex. Neurobiol Dis 101:1–7. https://doi.org/10.1016/j.nbd.2016.12.017
EbrahimAmini A, Bazzigaluppi P, Aquilino MS et al (2021) Neocortical in vivo focal and spreading potassium responses and the influence of astrocytic gap junctional coupling. Neurobiol Dis 147:105160. https://doi.org/10.1016/j.nbd.2020.105160
Deshpande T, Li T, Henning L et al (2020) Constitutive deletion of astrocytic connexins aggravates kainate-induced epilepsy. Glia 68:2136–2147. https://doi.org/10.1002/glia.23832
Chever O, Dossi E, Pannasch U et al (2016) Astroglial networks promote neuronal coordination. Sci Signal 9:ra6–ra6. https://doi.org/10.1126/scisignal.aad3066
Hösli L, Binini N, Ferrari KD et al (2022) Decoupling astrocytes in adult mice impairs synaptic plasticity and spatial learning. Cell Rep 38:110484. https://doi.org/10.1016/j.celrep.2022.110484
Löscher W, Friedman A (2020) Structural, Molecular, and Functional Alterations of the Blood-Brain Barrier during Epileptogenesis and Epilepsy: A Cause, Consequence, or Both? Int J Mol Sci 21:591. https://doi.org/10.3390/ijms21020591
van Vliet EA, Aronica E, Gorter JA (2015) Blood–brain barrier dysfunction, seizures and epilepsy. Semin Cell Dev Biol 38:26–34. https://doi.org/10.1016/j.semcdb.2014.10.003
Braganza O, Bedner P, Hüttmann K et al (2012) Albumin is taken up by hippocampal NG2 cells and astrocytes and decreases gap junction coupling. Albumin Blocks Gap Junction Coupling Epilepsia 53:1898–1906. https://doi.org/10.1111/j.1528-1167.2012.03665.x
Senatorov V, Friedman A, Milikovsky D et al (2019) Blood-brain barrier dysfunction in aging induces hyperactivation of TGFbeta signaling and chronic yet reversible neural dysfunction.Sci Transl Med15
van Vliet EA, da Costa Araujo S, Redeker S et al (2007) Blood-brain barrier leakage may lead to progression of temporal lobe epilepsy. Brain 130:521–534. https://doi.org/10.1093/brain/awl318
Bar-Klein G, Cacheaux LP, Kamintsky L et al (2014) Losartan prevents acquired epilepsy via TGF-β signaling suppression: Astrocytic TGF-β and Epilepsy. Ann Neurol 75:864–875. https://doi.org/10.1002/ana.24147
Cacheaux LP, Ivens S, David Y et al (2009) Transcriptome Profiling Reveals TGF- Signaling Involvement in Epileptogenesis. J Neurosci 29:8927–8935. https://doi.org/10.1523/JNEUROSCI.0430-09.2009
Ivens S, Kaufer D, Flores LP et al (2007) TGF- receptor-mediated albumin uptake into astrocytes is involved in neocortical epileptogenesis. Brain 130:535–547. https://doi.org/10.1093/brain/awl317
Weissberg I, Wood L, Kamintsky L et al (2015) Albumin induces excitatory synaptogenesis through astrocytic TGF-β/ALK5 signaling in a model of acquired epilepsy following blood–brain barrier dysfunction. Neurobiol Dis 78:115–125. https://doi.org/10.1016/j.nbd.2015.02.029
Frigerio F, Frasca A, Weissberg I et al (2012) Long-lasting pro-ictogenic effects induced in vivo by rat brain exposure to serum albumin in the absence of concomitant pathology: Albumin and Brain Excitability. Epilepsia 53:1887–1897. https://doi.org/10.1111/j.1528-1167.2012.03666.x
Greene C, Hanley N, Reschke CR et al (2022) Microvascular stabilization via blood-brain barrier regulation prevents seizure activity. Nat Commun 13:2003. https://doi.org/10.1038/s41467-022-29657-y
Henning L, Steinhäuser C, Bedner P (2021) Initiation of Experimental Temporal Lobe Epilepsy by Early Astrocyte Uncoupling Is Independent of TGFβR1/ALK5 Signaling. Front Neurol 12:660591. https://doi.org/10.3389/fneur.2021.660591
Tchekalarova JD, Ivanova NM, Pechlivanova DM et al (2014) Antiepileptogenic and neuroprotective effects of losartan in kainate model of temporal lobe epilepsy. Pharmacol Biochem Behav 127:27–36. https://doi.org/10.1016/j.pbb.2014.10.005
Zhang Y, Zhang M, Zhu W et al (2020) Role of Elevated Thrombospondin-1 in Kainic Acid-Induced Status Epilepticus. Neurosci Bull 36:263–276. https://doi.org/10.1007/s12264-019-00437-x
Zhang Y, Zhu W, Yu H et al (2019) P2Y4/TSP-1/TGF-β1/pSmad2/3 pathway contributes to acute generalized seizures induced by kainic acid. Brain Res Bull 149:106–119. https://doi.org/10.1016/j.brainresbull.2019.04.004
Danbolt NC (2001) Glutamate uptake. Prog Neurobiol 65:1–105. https://doi.org/10.1016/s0301-0082(00)00067-8
Rose CR, Felix L, Zeug A et al (2017) Astroglial Glutamate Signaling and Uptake in the Hippocampus. Front Mol Neurosci 10:451. https://doi.org/10.3389/fnmol.2017.00451
Schousboe A (2020) Astrocytic Metabolism Focusing on Glutamate Homeostasis: A Short Review Dedicated to Vittorio Gallo. Neurochem Res 45:522–525. https://doi.org/10.1007/s11064-019-02888-0
Sandhu MRS, Gruenbaum BF, Gruenbaum SE et al (2021) Astroglial Glutamine Synthetase and the Pathogenesis of Mesial Temporal Lobe Epilepsy. Front Neurol 12:665334. https://doi.org/10.3389/fneur.2021.665334
Bak LK, Schousboe A, Waagepetersen HS (2006) The glutamate/GABA-glutamine cycle: aspects of transport, neurotransmitter homeostasis and ammonia transfer. J Neurochem 98:641–653. https://doi.org/10.1111/j.1471-4159.2006.03913.x
During MJ, Spencer DD (1993) Extracellular hippocampal glutamate and spontaneous seizure in the conscious human brain. The Lancet 341:1607–1610. https://doi.org/10.1016/0140-6736(93)90754-5
Cavus I, Kasoff WS, Cassaday MP et al (2005) Extracellular metabolites in the cortex and hippocampus of epileptic patients. Ann Neurol 57:226–235. https://doi.org/10.1002/ana.20380
Boison D, Steinhäuser C (2018) Epilepsy and astrocyte energy metabolism. Glia 66:1235–1243. https://doi.org/10.1002/glia.23247
Mathern GW, Mendoza D, Lozada A et al (1999) Hippocampal GABA and glutamate transporter immunoreactivity in patients with temporal lobe epilepsy. Neurology 52:453–453. https://doi.org/10.1212/WNL.52.3.453
Proper EA, Hoogland G, Kappen SM et al (2002) Distribution of glutamate transporters in the hippocampus of patients with pharmaco-resistant temporal lobe epilepsy. Brain 125:32–43. https://doi.org/10.1093/brain/awf001
Sarac S, Afzal S, Broholm H et al (2009) Excitatory amino acid transporters EAAT-1 and EAAT-2 in temporal lobe and hippocampus in intractable temporal lobe epilepsy. APMIS 117:291–301. https://doi.org/10.1111/j.1600-0463.2009.02443.x
Tessler S, Danbolt NC, Faull RLM et al (1999) Expression of the glutamate transporters in human temporal lobe epilepsy. Neuroscience 88:1083–1091. https://doi.org/10.1016/S0306-4522(98)00301-7
Eid T, Thomas MJ, Spencer DD et al (2004) Loss of glutamine synthetase in the human epileptogenic hippocampus: possible mechanism for raised extracellular glutamate in mesial temporal lobe epilepsy. Lancet Lond Engl 363:28–37. https://doi.org/10.1016/s0140-6736(03)15166-5
Murphy-Royal C, Dupuis JP, Varela JA et al (2015) Surface diffusion of astrocytic glutamate transporters shapes synaptic transmission. Nat Neurosci 18:219–226. https://doi.org/10.1038/nn.3901
Armbruster M, Hanson E, Dulla CG (2016) Glutamate Clearance Is Locally Modulated by Presynaptic Neuronal Activity in the Cerebral Cortex. J Neurosci 36:10404–10415. https://doi.org/10.1523/JNEUROSCI.2066-16.2016
Pannasch U, Freche D, Dallérac G et al (2014) Connexin 30 sets synaptic strength by controlling astroglial synapse invasion. Nat Neurosci 17:549–558. https://doi.org/10.1038/nn.3662
Herde MK, Bohmbach K, Domingos C et al (2020) Local Efficacy of Glutamate Uptake Decreases with Synapse Size. Cell Rep 32:108182. https://doi.org/10.1016/j.celrep.2020.108182
Henneberger C, Bard L, Panatier A et al (2020) LTP Induction Boosts Glutamate Spillover by Driving Withdrawal of Perisynaptic Astroglia. Neuron 108:919–936e11. https://doi.org/10.1016/j.neuron.2020.08.030
Zeug A, Müller FE, Anders S et al (2018) Control of astrocyte morphology by Rho GTPases. Brain Res Bull 136:44–53. https://doi.org/10.1016/j.brainresbull.2017.05.003
Müller FE, Schade SK, Cherkas V et al (2021) Serotonin receptor 4 regulates hippocampal astrocyte morphology and function. Glia 69:872–889. https://doi.org/10.1002/glia.23933
İnan SY, Büyükafşar K (2008) Antiepileptic effects of two Rho-kinase inhibitors, Y-27632 and fasudil, in mice. Br J Pharmacol 155:44–51. https://doi.org/10.1038/bjp.2008.225
Jeon BT, Jeong EA, Park S-Y et al (2013) The Rho-Kinase (ROCK) Inhibitor Y-27632 Protects Against Excitotoxicity-Induced Neuronal Death In Vivo and In Vitro. Neurotox Res 23:238–248. https://doi.org/10.1007/s12640-012-9339-2
van der Hel WS, Notenboom RGE, Bos IWM et al (2005) Reduced glutamine synthetase in hippocampal areas with neuron loss in temporal lobe epilepsy. Neurology 64:326–333. https://doi.org/10.1212/01.WNL.0000149636.44660.99
Häberle J, Görg B, Rutsch F et al (2005) Congenital Glutamine Deficiency with Glutamine Synthetase Mutations. N Engl J Med 353:1926–1933. https://doi.org/10.1056/NEJMoa050456
Häberle J, Shahbeck N, Ibrahim K et al (2011) Natural course of glutamine synthetase deficiency in a 3year old patient. Mol Genet Metab 103:89–91. https://doi.org/10.1016/j.ymgme.2011.02.001
Ortinski PI, Dong J, Mungenast A et al (2010) Selective induction of astrocytic gliosis generates deficits in neuronal inhibition. Nat Neurosci 13:584–591. https://doi.org/10.1038/nn.2535
Tescarollo FC, Rombo DM, DeLiberto LK et al (2020) Role of Adenosine in Epilepsy and Seizures. J Caffeine Adenosine Res 10:45–60. https://doi.org/10.1089/caff.2019.0022
Beamer E, Kuchukulla M, Boison D, Engel T (2021) ATP and adenosine—Two players in the control of seizures and epilepsy development. Prog Neurobiol 204:102105. https://doi.org/10.1016/j.pneurobio.2021.102105
During MJ, Spencer DD (1992) Adenosine: A potential mediator of seizure arrest and postictal refractoriness. Ann Neurol 32:618–624. https://doi.org/10.1002/ana.410320504
Aronica E, Zurolo E, Iyer A et al (2011) Upregulation of adenosine kinase in astrocytes in experimental and human temporal lobe epilepsy. Epilepsia 52:1645–1655. https://doi.org/10.1111/j.1528-1167.2011.03115.x
Binder DK, Steinhäuser C (2021) Astrocytes and Epilepsy. Neurochem Res 46:2687–2695. https://doi.org/10.1007/s11064-021-03236-x
Young D, Fong DM, Lawlor PA et al (2014) Adenosine kinase, glutamine synthetase and EAAT2 as gene therapy targets for temporal lobe epilepsy. Gene Ther 21:1029–1040. https://doi.org/10.1038/gt.2014.82
Gouder N (2004) Overexpression of Adenosine Kinase in Epileptic Hippocampus Contributes to Epileptogenesis. J Neurosci 24:692–701. https://doi.org/10.1523/JNEUROSCI.4781-03.2004
Fedele DE, Gouder N, Güttinger M et al (2005) Astrogliosis in epilepsy leads to overexpression of adenosine kinase, resulting in seizure aggravation. Brain 128:2383–2395. https://doi.org/10.1093/brain/awh555
Shen H-Y, Sun H, Hanthorn MM et al (2014) Overexpression of adenosine kinase in cortical astrocytes and focal neocortical epilepsy in mice: Laboratory investigation. J Neurosurg 120:628–638. https://doi.org/10.3171/2013.10.JNS13918
Guttinger M, Fedele D, Koch P et al (2005) Suppression of Kindled Seizures by Paracrine Adenosine Release from Stem Cell-Derived Brain Implants. Epilepsia 46:1162–1169. https://doi.org/10.1111/j.1528-1167.2005.61804.x
Huber A, Padrun V, Déglon N et al (2001) Grafts of adenosine-releasing cells suppress seizures in kindling epilepsy. Proc Natl Acad Sci 98:7611–7616. https://doi.org/10.1073/pnas.131102898
Li T, Steinbeck JA, Lusardi T et al (2007) Suppression of kindling epileptogenesis by adenosine releasing stem cell-derived brain implants. Brain 130:1276–1288. https://doi.org/10.1093/brain/awm057
Williams-Karnesky RL, Sandau US, Lusardi TA et al (2013) Epigenetic changes induced by adenosine augmentation therapy prevent epileptogenesis. J Clin Invest 123:3552–3563. https://doi.org/10.1172/JCI65636
Sandau US, Yahya M, Bigej R et al (2019) Transient use of a systemic adenosine kinase inhibitor attenuates epilepsy development in mice. Epilepsia 60:615–625. https://doi.org/10.1111/epi.14674
Zhang Y, Chen K, Sloan SA et al (2014) An RNA-Sequencing Transcriptome and Splicing Database of Glia, Neurons, and Vascular Cells of the Cerebral Cortex. J Neurosci 34:11929–11947. https://doi.org/10.1523/JNEUROSCI.1860-14.2014
Chai H, Diaz-Castro B, Shigetomi E et al (2017) Neural Circuit-Specialized Astrocytes: Transcriptomic, Proteomic, Morphological, and Functional Evidence. Neuron 95:531–549e9. https://doi.org/10.1016/j.neuron.2017.06.029
Zhang Y, Sloan SA, Clarke LE et al (2016) Purification and Characterization of Progenitor and Mature Human Astrocytes Reveals Transcriptional and Functional Differences with Mouse. Neuron 89:37–53. https://doi.org/10.1016/j.neuron.2015.11.013
Araque A, Carmignoto G, Haydon PG et al (2014) Gliotransmitters travel in time and space. Neuron 81:728–739. https://doi.org/10.1016/j.neuron.2014.02.007
Rusakov DA, Bard L, Stewart MG, Henneberger C (2014) Diversity of astroglial functions alludes to subcellular specialisation. Trends Neurosci 37:228–242. https://doi.org/10.1016/j.tins.2014.02.008
Navarrete M, Perea G, Maglio L et al (2013) Astrocyte Calcium Signal and Gliotransmission in Human Brain Tissue. Cereb Cortex 23:1240–1246. https://doi.org/10.1093/cercor/bhs122
Tang F-R, Lee W-L, Yeo TT (2002) Expression of the group I metabotropic glutamate receptor in the hippocampus of patients with mesial temporal lobe epilepsy. J Neurocytol 30:403–411. https://doi.org/10.1023/A:1015065626262
Kandratavicius L, Rosa-Neto P, Monteiro MR et al (2013) Distinct increased metabotropic glutamate receptor type 5 (mGluR5) in temporal lobe epilepsy with and without hippocampal sclerosis. Hippocampus 23:1212–1230. https://doi.org/10.1002/hipo.22160
Aronica E, Gorter JA, Ijlst-Keizers H et al (2003) Expression and functional role of mGluR3 and mGluR5 in human astrocytes and glioma cells: opposite regulation of glutamate transporter proteins. Eur J Neurosci 17:2106–2118. https://doi.org/10.1046/j.1460-9568.2003.02657.x
Notenboom RGE, Hampson DR, Jansen GH et al (2006) Up-regulation of hippocampal metabotropic glutamate receptor 5 in temporal lobe epilepsy patients. Brain 129:96–107. https://doi.org/10.1093/brain/awh673
Lee TS, Eid T, Mane S et al (2004) Aquaporin-4 is increased in the sclerotic hippocampus in human temporal lobe epilepsy. Acta Neuropathol (Berl) 108:493–502. https://doi.org/10.1007/s00401-004-0910-7
Eid T, Lee T-SW, Thomas MJ et al (2005) Loss of perivascular aquaporin 4 may underlie deficient water and K + homeostasis in the human epileptogenic hippocampus. Proc Natl Acad Sci 102:1193–1198. https://doi.org/10.1073/pnas.0409308102
Alvestad S, Hammer J, Hoddevik EH et al (2013) Mislocalization of AQP4 precedes chronic seizures in the kainate model of temporal lobe epilepsy. Epilepsy Res 105:30–41. https://doi.org/10.1016/j.eplepsyres.2013.01.006
Bankstahl M, Breuer H, Leiter I et al (2018) Blood–Brain Barrier Leakage during Early Epileptogenesis Is Associated with Rapid Remodeling of the Neurovascular Unit. https://doi.org/10.1523/ENEURO.0123-18.2018. eneuro 5:ENEURO.0123-18.2018
Hubbard JA, Szu JI, Yonan JM, Binder DK (2016) Regulation of astrocyte glutamate transporter-1 (GLT1) and aquaporin-4 (AQP4) expression in a model of epilepsy. Exp Neurol 283:85–96. https://doi.org/10.1016/j.expneurol.2016.05.003
Lee DJ, Hsu MS, Seldin MM et al (2012) Decreased expression of the glial water channel aquaporin-4 in the intrahippocampal kainic acid model of epileptogenesis. Exp Neurol 235:246–255. https://doi.org/10.1016/j.expneurol.2012.02.002
Szu JI, Chaturvedi S, Patel DD, Binder DK (2020) Aquaporin-4 Dysregulation in a Controlled Cortical Impact Injury Model of Posttraumatic Epilepsy. Neuroscience 428:140–153. https://doi.org/10.1016/j.neuroscience.2019.12.006
Steinhäuser C, Grunnet M, Carmignoto G (2016) Crucial role of astrocytes in temporal lobe epilepsy. Neuroscience 323:157–169. https://doi.org/10.1016/j.neuroscience.2014.12.047
Strohschein S, Hüttmann K, Gabriel S et al (2011) Impact of aquaporin-4 channels on K + buffering and gap junction coupling in the hippocampus. Glia 59:973–980. https://doi.org/10.1002/glia.21169
Funding
Work in our laboratories is currently supported by the German Research Foundation (DFG; SFB1089 B03, FOR2795 P02, HE6949/5, and HE6949/8 to C.H.; DFG; STE552/4 and BMBF; 01DN20001 to C.S.).
Open Access funding enabled and organized by Projekt DEAL.
Author information
Authors and Affiliations
Contributions
All authors contributed equally to this work.
Corresponding authors
Ethics declarations
Competing Interests
All authors declare no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Henning, L., Unichenko, P., Bedner, P. et al. Overview Article Astrocytes as Initiators of Epilepsy. Neurochem Res 48, 1091–1099 (2023). https://doi.org/10.1007/s11064-022-03773-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-022-03773-z