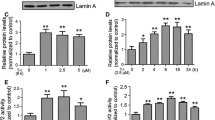
Abstract
Parkinson’s disease (PD) is a prevalent, progressive, neurodegenerative disorder with no known cure. Oxidative stress has been found to play a significant role in its etiology, and the search for novel neuroprotective compounds that actively prevent disease progression is currently ongoing. Dithiolethiones are a group of sulfur-containing heterocyclic compounds found in cruciferous vegetables. Using the 6-hydroxydopamine (6-OHDA) model of PD, we tested a previously identified disubstituted dithiolethione 5-amino-3-thioxo-3H-(1,2) dithiole-4-carboxylic acid ethyl ester (ACDT) for its neuroprotective potential. Pretreatment of SH-SY5Y cells with ACDT led to a time- and concentration-dependent induction of the antioxidant glutathione (GSH). ACDT also diminished 6-OHDA-induced cell death, lactate dehydrogenase release, elevation of caspase 3/7 activity, and increase in levels of reactive oxygen species. Inhibition of the GSH-synthesizing enzyme glutamate-cysteine ligase catalytic subunit (GCLC) led a corresponding dissipation of ACDT’s neuroprotective effects, hence underlining the importance of GSH in ACDT’s neuroprotective response. ACDT caused the stabilization and nuclear translocation of nuclear factor erythroid-2 related factor (Nrf2), resulting in increased protein expression of the phase II enzyme NADPH:quinone oxidoreductase 1 (NQO1), and the excitatory amino acid cysteine membrane transporter (EAAT3). Interestingly, no changes in the levels of other Nrf2-dependent molecules including GCLC were observed, indicating the possible involvement of additional alternate mechanisms behind ACDT’s GSH-inducing property. Collectively, the data demonstrated ACDT to be a promising new dithiolethione for the treatment of PD, with two modifiable functional groups offering additional avenues for enhanced pharmacological application.







Similar content being viewed by others
References
Postuma RB, Berg D, Stern M, Poewe W, Olanow CW, Oertel W, Obeso J, Marek K, Litvan I, Lang AE, Halliday G, Goetz CG, Gasser T, Dubois B, Chan P, Bloem BR, Adler CH, Deuschl G (2015) MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 30(12):1591–1601. https://doi.org/10.1002/mds.26424
Spina MB, Cohen G (1989) Dopamine turnover and glutathione oxidation: implications for Parkinson disease. Proc Natl Acad Sci USA 86(4):1398–1400
Ben-Shachar D, Youdim MB (1993) Iron, melanin and dopamine interaction: relevance to Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 17(1):139–150
Gerlach M, Double KL, Ben-Shachar D, Zecca L, Youdim MB, Riederer P (2003) Neuromelanin and its interaction with iron as a potential risk factor for dopaminergic neurodegeneration underlying Parkinson’s disease. Neurotox Res 5(1–2):35–44
Gotz ME, Double K, Gerlach M, Youdim MB, Riederer P (2004) The relevance of iron in the pathogenesis of Parkinson’s disease. Ann N Y Acad Sci 1012:193–208
Enochs WS, Sarna T, Zecca L, Riley PA, Swartz HM (1994) The roles of neuromelanin, binding of metal ions, and oxidative cytotoxicity in the pathogenesis of Parkinson’s disease: a hypothesis. J Neural Transm Park Dis Dement Sect 7(2):83–100
Dexter DT, Carter CJ, Wells FR, Javoy-Agid F, Agid Y, Lees A, Jenner P, Marsden CD (1989) Basal lipid peroxidation in substantia nigra is increased in Parkinson’s disease. J Neurochem 52(2):381–389
Sherer TB, Betarbet R, Greenamyre JT (2002) Environment, mitochondria, and Parkinson’s disease. Neuroscientist 8(3):192–197. https://doi.org/10.1177/1073858402008003004
Spencer JP, Jenner A, Aruoma OI, Evans PJ, Kaur H, Dexter DT, Jenner P, Lees AJ, Marsden DC, Halliwell B (1994) Intense oxidative DNA damage promoted by L-dopa and its metabolites. Implications for neurodegenerative disease. FEBS Lett 353(3):246–250
Sugawara T, Chan PH (2003) Reactive oxygen radicals and pathogenesis of neuronal death after cerebral ischemia. Antioxid Redox Signal 5(5):597–607. https://doi.org/10.1089/152308603770310266
Alam ZI, Jenner A, Daniel SE, Lees AJ, Cairns N, Marsden CD, Jenner P, Halliwell B (1997) Oxidative DNA damage in the parkinsonian brain: an apparent selective increase in 8-hydroxyguanine levels in substantia nigra. J Neurochem 69(3):1196–1203
Castellani RJ, Perry G, Siedlak SL, Nunomura A, Shimohama S, Zhang J, Montine T, Sayre LM, Smith MA (2002) Hydroxynonenal adducts indicate a role for lipid peroxidation in neocortical and brainstem Lewy bodies in humans. Neurosci Lett 319(1):25–28
Perry TL, Yong VW (1986) Idiopathic Parkinson’s disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci Lett 67(3):269–274
Siegel D, Bolton EM, Burr JA, Liebler DC, Ross D (1997) The reduction of alpha-tocopherolquinone by human NAD(P)H: quinone oxidoreductase: the role of alpha-tocopherolhydroquinone as a cellular antioxidant. Mol Pharmacol 52(2):300–305
Spencer JP, Jenner P, Daniel SE, Lees AJ, Marsden DC, Halliwell B (1998) Conjugates of catecholamines with cysteine and GSH in Parkinson’s disease: possible mechanisms of formation involving reactive oxygen species. J Neurochem 71(5):2112–2122
Ross D (2004) Quinone reductases multitasking in the metabolic world. Drug Metab Rev 36(3–4):639–654. https://doi.org/10.1081/DMR-200033465
Ross D, Kepa JK, Winski SL, Beall HD, Anwar A, Siegel D (2000) NAD(P)H:quinone oxidoreductase 1 (NQO1): chemoprotection, bioactivation, gene regulation and genetic polymorphisms. Chem Biol Interact 129(1–2):77–97
Siegel D, Gustafson DL, Dehn DL, Han JY, Boonchoong P, Berliner LJ, Ross D (2004) NAD(P)H:quinone oxidoreductase 1: role as a superoxide scavenger. Mol Pharmacol 65(5):1238–1247. https://doi.org/10.1124/mol.65.5.1238
Pearce RK, Owen A, Daniel S, Jenner P, Marsden CD (1997) Alterations in the distribution of glutathione in the substantia nigra in Parkinson’s disease. J Neural Transm 104(6–7):661–677. https://doi.org/10.1007/BF01291884
Mytilineou C, Kramer BC, Yabut JA (2002) Glutathione depletion and oxidative stress. Parkinsonism Relat Disord 8(6):385–387
Perry TL, Godin DV, Hansen S (1982) Parkinson’s disease: a disorder due to nigral glutathione deficiency? Neurosci Lett 33(3):305–310
Spencer JP, Jenner P, Halliwell B (1995) Superoxide-dependent depletion of reduced glutathione by L-DOPA and dopamine. Relevance to Parkinson’s disease. NeuroReport 6(11):1480–1484
Jenner P, Dexter DT, Sian J, Schapira AH, Marsden CD (1992) Oxidative stress as a cause of nigral cell death in Parkinson’s disease and incidental Lewy body disease. The Royal Kings and Queens Parkinson’s Disease Research Group. Ann Neurol 32(Suppl):S82–S87
Ahlskog JE (1990) Parkinson’s disease: new treatment strategies. Compr Ther 16(12):41–46
Ballaz S, Morales I, Rodriguez M, Obeso JA (2013) Ascorbate prevents cell death from prolonged exposure to glutamate in an in vitro model of human dopaminergic neurons. J Neurosci Res 91(12):1609–1617. https://doi.org/10.1002/jnr.23276
Kittur S, Wilasrusmee S, Pedersen WA, Mattson MP, Straube-West K, Wilasrusmee C, Lubelt B, Kittur DS (2002) Neurotrophic and neuroprotective effects of milk thistle (Silybum marianum) on neurons in culture. J Mol Neurosci 18(3):265–269
Korytowski W, Sarna T, Zarba M (1995) Antioxidant action of neuromelanin: the mechanism of inhibitory effect on lipid peroxidation. Arch Biochem Biophys 319(1):142–148
Sechi G, Deledda MG, Bua G, Satta WM, Deiana GA, Pes GM, Rosati G (1996) Reduced intravenous glutathione in the treatment of early Parkinson’s disease. Prog Neuropsychopharmacol Biol Psychiatry 20(7):1159–1170
Sharma A, Kaur P, Kumar V, Gill KD (2007) Attenuation of 1-methyl-4-phenyl-1, 2,3,6-tetrahydropyridine induced nigrostriatal toxicity in mice by N-acetyl cysteine. Cell Mol Biol 53(1):48–55
Ebadi M, Srinivasan SK, Baxi MD (1996) Oxidative stress and antioxidant therapy in Parkinson’s disease. Prog Neurobiol 48(1):1–19
Ratnam DV, Ankola DD, Bhardwaj V, Sahana DK, Kumar MN (2006) Role of antioxidants in prophylaxis and therapy: a pharmaceutical perspective. J Control Release 113(3):189–207. https://doi.org/10.1016/j.jconrel.2006.04.015
Yang KY, du Hwang H, Yousaf AM, Kim DW, Shin YJ, Bae ON, Kim YI, Kim JO, Yong CS, Choi HG (2013) Silymarin-loaded solid nanoparticles provide excellent hepatic protection: physicochemical characterization and in vivo evaluation. Int J Nanomed 8:3333–3343. https://doi.org/10.2147/IJN.S50683
Cao Z, Hardej D, Trombetta LD, Trush MA, Li Y (2003) Induction of cellular glutathione and glutathione S-transferase by 3H-1,2-dithiole-3-thione in rat aortic smooth muscle A10 cells: protection against acrolein-induced toxicity. Atherosclerosis 166(2):291–301
Kwak MK, Egner PA, Dolan PM, Ramos-Gomez M, Groopman JD, Itoh K, Yamamoto M, Kensler TW (2001) Role of phase 2 enzyme induction in chemoprotection by dithiolethiones. Mutat Res 480–481:305–315
Jia Z, Misra BR, Zhu H, Li Y, Misra HP (2009) Upregulation of cellular glutathione by 3H-1,2-dithiole-3-thione as a possible treatment strategy for protecting against acrolein-induced neurocytotoxicity. Neurotoxicology 30(1):1–9. https://doi.org/10.1016/j.neuro.2008.11.007
Jia Z, Zhu H, Misra HP, Li Y (2008) Potent induction of total cellular GSH and NQO1 as well as mitochondrial GSH by 3H-1,2-dithiole-3-thione in SH-SY5Y neuroblastoma cells and primary human neurons: protection against neurocytotoxicity elicited by dopamine, 6-hydroxydopamine, 4-hydroxy-2-nonenal, or hydrogen peroxide. Brain Res 1197:159–169. https://doi.org/10.1016/j.brainres.2007.12.044
Kuo PC, Brown DA, Scofield BA, Yu IC, Chang FL, Wang PY, Yen JH (2016) 3H-1,2-dithiole-3-thione as a novel therapeutic agent for the treatment of experimental autoimmune encephalomyelitis. Brain Behav Immun 57:173–186. https://doi.org/10.1016/j.bbi.2016.03.015
Kuo PC, Brown DA, Scofield BA, Paraiso HC, Wang PY, Yu IC, Yen JH (2018) Dithiolethione ACDT suppresses neuroinflammation and ameliorates disease severity in experimental autoimmune encephalomyelitis. Brain Behav Immun 70:76–87. https://doi.org/10.1016/j.bbi.2018.03.010
Kuo PC, Yu IC, Scofield BA, Brown DA, Curfman ET, Paraiso HC, Chang FL, Yen JH (2017) 3H-1,2-Dithiole-3-thione as a novel therapeutic agent for the treatment of ischemic stroke through Nrf2 defense pathway. Brain Behav Immun 62:180–192. https://doi.org/10.1016/j.bbi.2017.01.018
Cui Y, Ma S, Zhang C, Li D, Yang B, Lv P, Xing Q, Huang T, Yang GL, Cao W, Guan F (2018) Pharmacological activation of the Nrf2 pathway by 3H-1, 2-dithiole-3-thione is neuroprotective in a mouse model of Alzheimer disease. Behav Brain Res 336:219–226. https://doi.org/10.1016/j.bbr.2017.09.011
Wang L, Wang M, Hu J, Shen W, Hu J, Yao Y, Wang X, Afzal CM, Ma R, Li G (2017) Protective effect of 3H-1, 2-dithiole-3-thione on cellular model of Alzheimer’s disease involves Nrf2/ARE signaling pathway. Eur J Pharmacol 795:115–123. https://doi.org/10.1016/j.ejphar.2016.12.013
Brown DA, Betharia S, Yen JH, Kuo PC, Mistry H (2016) Further structure-activity relationships study of substituted dithiolethiones as glutathione-inducing neuroprotective agents. Chem Cent J 10:64. https://doi.org/10.1186/s13065-016-0210-z
Rondon-Ortiz AN, Lino Cardenas CL, Martinez-Malaga J, Gonzales-Urday AL, Gugnani KS, Bohlke M, Maher TJ, Pino-Figueroa AJ (2017) High concentrations of rosiglitazone reduce mRNA and protein levels of LRP1 in HepG2 cells. Front Pharmacol 8:772. https://doi.org/10.3389/fphar.2017.00772
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, Pietzsch T, Preibisch S, Rueden C, Saalfeld S, Schmid B, Tinevez JY, White DJ, Hartenstein V, Eliceiri K, Tomancak P, Cardona A (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9(7):676–682. https://doi.org/10.1038/nmeth.2019
Kumar R, Agarwal AK, Seth PK (1995) Free radical-generated neurotoxicity of 6-hydroxydopamine. J Neurochem 64(4):1703–1707
Soto-Otero R, Mendez-Alvarez E, Hermida-Ameijeiras A, Munoz-Patino AM, Labandeira-Garcia JL (2000) Autoxidation and neurotoxicity of 6-hydroxydopamine in the presence of some antioxidants: potential implication in relation to the pathogenesis of Parkinson’s disease. J Neurochem 74(4):1605–1612
Raichle ME, Gusnard DA (2002) Appraising the brain’s energy budget. Proc Natl Acad Sci USA 99(16):10237–10239. https://doi.org/10.1073/pnas.172399499
Coles LD, Tuite PJ, Oz G, Mishra UR, Kartha RV, Sullivan KM, Cloyd JC, Terpstra M (2018) Repeated-dose oral N-acetylcysteine in Parkinson’s disease: pharmacokinetics and effect on brain glutathione and oxidative stress. J Clin Pharmacol 58(2):158–167. https://doi.org/10.1002/jcph.1008
Fariss MW, Zhang JG (2003) Vitamin E therapy in Parkinson’s disease. Toxicology 189(1–2):129–146
Matthews RT, Ferrante RJ, Klivenyi P, Yang L, Klein AM, Mueller G, Kaddurah-Daouk R, Beal MF (1999) Creatine and cyclocreatine attenuate MPTP neurotoxicity. Exp Neurol 157(1):142–149. https://doi.org/10.1006/exnr.1999.7049
Mazzio E, Huber J, Darling S, Harris N, Soliman KF (2001) Effect of antioxidants on L-glutamate and N-methyl-4-phenylpyridinium ion induced-neurotoxicity in PC12 cells. Neurotoxicology 22(2):283–288
Mischley LK, Lau RC, Shankland EG, Wilbur TK, Padowski JM (2017) Phase IIb study of intranasal glutathione in Parkinson’s disease. J Parkinsons Dis 7(2):289–299. https://doi.org/10.3233/JPD-161040
Stull ND, Polan DP, Iacovitti L (2002) Antioxidant compounds protect dopamine neurons from death due to oxidative stress in vitro. Brain Res 931(2):181–185
Zhu ZG, Sun MX, Zhang WL, Wang WW, Jin YM, Xie CL (2017) The efficacy and safety of coenzyme Q10 in Parkinson’s disease: a meta-analysis of randomized controlled trials. Neurol Sci 38(2):215–224. https://doi.org/10.1007/s10072-016-2757-9
Peng X, Li Y (2002) Induction of cellular glutathione-linked enzymes and catalase by the unique chemoprotective agent, 3H-1,2-dithiole-3-thione in rat cardiomyocytes affords protection against oxidative cell injury. Pharmacol Res 45(6):491–497
Vargas M, Lamb JG, Franklin MR (1998) Phase II-selective induction of hepatic drug-metabolizing enzymes by oltipraz -5-(2-pyrazinyl)-4-methyl-1,2-dithiol-3-thione-, 1,7-phenanthroline, and 2,2′-dipyridyl in rats is not accompanied by induction of intestinal enzymes. Drug Metab Dispos 26(2):91–97
Zhang Y, Munday R (2008) Dithiolethiones for cancer chemoprevention: where do we stand? Mol Cancer Ther 7(11):3470–3479. https://doi.org/10.1158/1535-7163.MCT-08-0625
Brown DA, Betharia S, Yen JH, Tran Q, Mistry H, Smith K (2014) Synthesis and structure-activity relationships study of dithiolethiones as inducers of glutathione in the SH-SY5Y neuroblastoma cell line. Bioorg Med Chem Lett 24(24):5829–5831. https://doi.org/10.1016/j.bmcl.2014.10.005
Dong J, Yan D, Chen SY (2011) Stabilization of Nrf2 protein by D3T provides protection against ethanol-induced apoptosis in PC12 cells. PLoS ONE 6(2):e16845. https://doi.org/10.1371/journal.pone.0016845
Liu XY, Li CY, Bu H, Li Z, Li B, Sun MM, Guo YS, Zhang L, Ren WB, Fan ZL, Wu DX, Wu SY (2008) The neuroprotective potential of phase II enzyme inducer on motor neuron survival in traumatic spinal cord injury in vitro. Cell Mol Neurobiol 28(5):769–779. https://doi.org/10.1007/s10571-007-9219-0
Munday R, Zhang Y, Paonessa JD, Munday CM, Wilkins AL, Babu J (2010) Synthesis, biological evaluation, and structure-activity relationships of dithiolethiones as inducers of cytoprotective phase 2 enzymes. J Med Chem 53(12):4761–4767. https://doi.org/10.1021/jm100425v
Yu Z, Shao W, Chiang Y, Foltz W, Zhang Z, Ling W, Fantus IG, Jin T (2011) Oltipraz upregulates the nuclear factor (erythroid-derived 2)-like 2 [corrected](NRF2) antioxidant system and prevents insulin resistance and obesity induced by a high-fat diet in C57BL/6 J mice. Diabetologia 54(4):922–934. https://doi.org/10.1007/s00125-010-2001-8
McMahon M, Itoh K, Yamamoto M, Hayes JD (2003) Keap1-dependent proteasomal degradation of transcription factor Nrf2 contributes to the negative regulation of antioxidant response element-driven gene expression. J Biol Chem 278(24):21592–21600. https://doi.org/10.1074/jbc.M300931200
Nguyen T, Sherratt PJ, Nioi P, Yang CS, Pickett CB (2005) Nrf2 controls constitutive and inducible expression of ARE-driven genes through a dynamic pathway involving nucleocytoplasmic shuttling by Keap1. J Biol Chem 280(37):32485–32492. https://doi.org/10.1074/jbc.M503074200
Wakabayashi N, Dinkova-Kostova AT, Holtzclaw WD, Kang MI, Kobayashi A, Yamamoto M, Kensler TW, Talalay P (2004) Protection against electrophile and oxidant stress by induction of the phase 2 response: fate of cysteines of the Keap1 sensor modified by inducers. Proc Natl Acad Sci USA 101(7):2040–2045. https://doi.org/10.1073/pnas.0307301101
Zhang DD, Hannink M (2003) Distinct cysteine residues in Keap1 are required for Keap1-dependent ubiquitination of Nrf2 and for stabilization of Nrf2 by chemopreventive agents and oxidative stress. Mol Cell Biol 23(22):8137–8151
Zhu H, Itoh K, Yamamoto M, Zweier JL, Li Y (2005) Role of Nrf2 signaling in regulation of antioxidants and phase 2 enzymes in cardiac fibroblasts: protection against reactive oxygen and nitrogen species-induced cell injury. FEBS Lett 579(14):3029–3036. https://doi.org/10.1016/j.febslet.2005.04.058
Holland R, Navamal M, Velayutham M, Zweier JL, Kensler TW, Fishbein JC (2009) Hydrogen peroxide is a second messenger in phase 2 enzyme induction by cancer chemopreventive dithiolethiones. Chem Res Toxicol 22(8):1427–1434. https://doi.org/10.1021/tx900110n
Petzer JP, Navamal M, Johnson JK, Kwak MK, Kensler TW, Fishbein JC (2003) Phase 2 enzyme induction by the major metabolite of oltipraz. Chem Res Toxicol 16(11):1463–1469. https://doi.org/10.1021/tx034154e
Xie HR, Hu LS, Li GY (2010) SH-SY5Y human neuroblastoma cell line: in vitro cell model of dopaminergic neurons in Parkinson’s disease. Chin Med J 123(8):1086–1092
Smythies J (1999) The neurotoxicity of glutamate, dopamine, iron and reactive oxygen species: functional interrelationships in health and disease: a review-discussion. Neurotox Res 1(1):27–39
Pazdro R, Burgess JR (2012) The antioxidant 3H-1,2-dithiole-3-thione potentiates advanced glycation end-product-induced oxidative stress in SH-SY5Y cells. Exp Diabetes Res 2012:137607. https://doi.org/10.1155/2012/137607
Zhang C, Xie L, Guan F, Cui Y (2018) 3H-1,2-dithiole-3-thione protects PC12 cells against amyloid beta 1-42 (Abeta1-42) induced apoptosis via activation of the ERK1/2 pathway. Life Sci 213:74–81. https://doi.org/10.1016/j.lfs.2018.10.025
Zhu H, Zhang L, Itoh K, Yamamoto M, Ross D, Trush MA, Zweier JL, Li Y (2006) Nrf2 controls bone marrow stromal cell susceptibility to oxidative and electrophilic stress. Free Radic Biol Med 41(1):132–143. https://doi.org/10.1016/j.freeradbiomed.2006.03.020
Zhu H, Zhang L, Trush MA, Li Y (2007) Upregulation of endogenous glutathione system by 3H-1,2-dithiole-3-thione in pancreatic RINm5F beta-cells as a novel strategy for protecting against oxidative beta-cell injury. Free Radic Res 41(2):242–250. https://doi.org/10.1080/10715760601009586
Ikeda Y, Tsuji S, Satoh A, Ishikura M, Shirasawa T, Shimizu T (2008) Protective effects of astaxanthin on 6-hydroxydopamine-induced apoptosis in human neuroblastoma SH-SY5Y cells. J Neurochem 107(6):1730–1740. https://doi.org/10.1111/j.1471-4159.2008.05743.x
Dong J, Sulik KK, Chen SY (2008) Nrf2-mediated transcriptional induction of antioxidant response in mouse embryos exposed to ethanol in vivo: implications for the prevention of fetal alcohol spectrum disorders. Antioxid Redox Signal 10(12):2023–2033. https://doi.org/10.1089/ars.2007.2019
Manandhar S, Cho JM, Kim JA, Kensler TW, Kwak MK (2007) Induction of Nrf2-regulated genes by 3H-1, 2-dithiole-3-thione through the ERK signaling pathway in murine keratinocytes. Eur J Pharmacol 577(1–3):17–27. https://doi.org/10.1016/j.ejphar.2007.08.018
Escartin C, Won SJ, Malgorn C, Auregan G, Berman AE, Chen PC, Deglon N, Johnson JA, Suh SW, Swanson RA (2011) Nuclear factor erythroid 2-related factor 2 facilitates neuronal glutathione synthesis by upregulating neuronal excitatory amino acid transporter 3 expression. J Neurosci 31(20):7392–7401. https://doi.org/10.1523/JNEUROSCI.6577-10.2011
Sasaki H, Sato H, Kuriyama-Matsumura K, Sato K, Maebara K, Wang H, Tamba M, Itoh K, Yamamoto M, Bannai S (2002) Electrophile response element-mediated induction of the cystine/glutamate exchange transporter gene expression. J Biol Chem 277(47):44765–44771. https://doi.org/10.1074/jbc.M208704200
Jing X, Shi H, Zhang C, Ren M, Han M, Wei X, Zhang X, Lou H (2015) Dimethyl fumarate attenuates 6-OHDA-induced neurotoxicity in SH-SY5Y cells and in animal model of Parkinson’s disease by enhancing Nrf2 activity. Neuroscience 286:131–140. https://doi.org/10.1016/j.neuroscience.2014.11.047
Huang HC, Nguyen T, Pickett CB (2002) Phosphorylation of Nrf2 at Ser-40 by protein kinase C regulates antioxidant response element-mediated transcription. J Biol Chem 277(45):42769–42774. https://doi.org/10.1074/jbc.M206911200
Koo JH, Lee WH, Lee CG, Kim SG (2012) Fyn inhibition by cycloalkane-fused 1,2-dithiole-3-thiones enhances antioxidant capacity and protects mitochondria from oxidative injury. Mol Pharmacol 82(1):27–36. https://doi.org/10.1124/mol.111.077149
Lee JM, Hanson JM, Chu WA, Johnson JA (2001) Phosphatidylinositol 3-kinase, not extracellular signal-regulated kinase, regulates activation of the antioxidant-responsive element in IMR-32 human neuroblastoma cells. J Biol Chem 276(23):20011–20016. https://doi.org/10.1074/jbc.M100734200
Yu R, Lei W, Mandlekar S, Weber MJ, Der CJ, Wu J, Kong AN (1999) Role of a mitogen-activated protein kinase pathway in the induction of phase II detoxifying enzymes by chemicals. J Biol Chem 274(39):27545–27552
Yu R, Mandlekar S, Lei W, Fahl WE, Tan TH, Kong AN (2000) p38 mitogen-activated protein kinase negatively regulates the induction of phase II drug-metabolizing enzymes that detoxify carcinogens. J Biol Chem 275(4):2322–2327
Nguyen T, Sherratt PJ, Huang HC, Yang CS, Pickett CB (2003) Increased protein stability as a mechanism that enhances Nrf2-mediated transcriptional activation of the antioxidant response element. Degradation of Nrf2 by the 26 S proteasome. J Biol Chem 278(7):4536–4541. https://doi.org/10.1074/jbc.m207293200
Munday R, Munday CM (2004) Induction of phase II enzymes by 3H-1,2-dithiole-3-thione: dose-response study in rats. Carcinogenesis 25(9):1721–1725. https://doi.org/10.1093/carcin/bgh162
Zheng Y, Ji X, Ji K, Wang B (2015) Hydrogen sulfide prodrugs—a review. Acta Pharm Sin B 5(5):367–377. https://doi.org/10.1016/j.apsb.2015.06.004
Yang G, Zhao K, Ju Y, Mani S, Cao Q, Puukila S, Khaper N, Wu L, Wang R (2013) Hydrogen sulfide protects against cellular senescence via S-sulfhydration of Keap1 and activation of Nrf2. Antioxid Redox Signal 18(15):1906–1919. https://doi.org/10.1089/ars.2012.4645
Kimura Y, Kimura H (2004) Hydrogen sulfide protects neurons from oxidative stress. FASEB J 18(10):1165–1167. https://doi.org/10.1096/fj.04-1815fje
Chen WL, Niu YY, Jiang WZ, Tang HL, Zhang C, Xia QM, Tang XQ (2015) Neuroprotective effects of hydrogen sulfide and the underlying signaling pathways. Rev Neurosci 26(2):129–142. https://doi.org/10.1515/revneuro-2014-0051
Meister A, Anderson ME (1983) Glutathione. Annu Rev Biochem 52:711–760. https://doi.org/10.1146/annurev.bi.52.070183.003431
Zhang H, Liu H, Dickinson DA, Liu RM, Postlethwait EM, Laperche Y, Forman HJ (2006) gamma-Glutamyl transpeptidase is induced by 4-hydroxynonenal via EpRE/Nrf2 signaling in rat epithelial type II cells. Free Radic Biol Med 40(8):1281–1292. https://doi.org/10.1016/j.freeradbiomed.2005.11.005
Alroughani R, Ahmed SF, Behbehani R, Al-Hashel J (2017) Effectiveness and safety of dimethyl fumarate treatment in relapsing multiple sclerosis patients: real-world evidence. Neurol Ther 6(2):189–196. https://doi.org/10.1007/s40120-017-0080-x
Acknowledgements
This work was supported by Manchester University College of Pharmacy, Natural & Health Science, Fort Wayne, IN, and MCPHS University School of Pharmacy, Boston, MA. The authors wish to thank Evan Beakas, Rosary Ajaelu, and Iman Aoude for their participation in the project.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Betharia, S., Rondόn-Ortiz, A.N. & Brown, D.A. Disubstituted Dithiolethione ACDT Exerts Neuroprotective Effects Against 6-Hydroxydopamine-Induced Oxidative Stress in SH-SY5Y Cells. Neurochem Res 44, 1878–1892 (2019). https://doi.org/10.1007/s11064-019-02823-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11064-019-02823-3