Summary
Depletion of reduced glutathione occurs in the substantia nigra in Parkinson's disease and in incidental Lewy body disease (presymptomatic Parkinson's disease) which may implicate oxidative stress in the neurode-generative process. In this study mercury orange fluorescent staining and immunostaining with an antibody to reduced glutathione have been used to determine the distribution of reduced glutathione in the substantia nigra in Parkinson's disease compared with normal individuals.
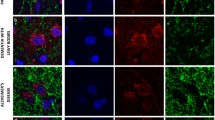
Mercury orange staining showed moderate background levels of fluorescence in the neuropil in both control and Parkinson's disease substantia nigra and localised reduced glutathione to the somata of melanized nigral neurons and glial elements of the neuropil. Neuronal nuclei revealed a relative lack of fluorescence after mercury orange staining. There was a significant depletion of reduced glutathione in surviving neurons in Parkinson's disease compared to nerve cell populations in control tissue. Mercury orange fluorescence indicated a high concentration of reduced glutathione in a subpopulation of non-neuronal cells, most likely astrocytes or microglia.
Immunohistochemical examination of nigral tissue from the same Parkinson's disease and control patients with an antibody to glutathione showed staining in neuronal perikarya and axonodendritic processes of melanized nigral neurons which was generally most intense in control neurons. Moderately intense staining of the background neuropil, most prominent in control nigras, and staining of capillary walls was also detected. Intense staining was seen in cells with the morphological features of glial cells in both control and PD nigra.
These data show a significant presence of reduced glutathione in the cell bodies and axons of nigral neurons. They are in agreement with biochemical studies showing depletion of reduced glutathione in substantia nigra in Parkinson's disease, and indicate a significant loss of neuronal reduced glutathione in surviving nigral neurons in Parkinson's disease.
Similar content being viewed by others
Abbreviations
- GFAP :
-
glial fibrillary acidic protein
- GSH :
-
reduced glutathione
- GSSG :
-
oxidized glutathione
- ILBD :
-
incidental Lewy body disease
- PBS :
-
phosphate-buffered saline
- PD :
-
Parkinson's disease
References
Amara A, Coussemacq M, Geffard M (1994) Antibodies to reduced glutathione. Brain Res 659: 237–242
Asghar K, Reddy BG, Krishna G (1975) Histochemical localisation of glutathione in tissues. J Histochem Cytochem 23: 774–779
Benzi G, Pastoris O, Marzatico F, Villa RF (1992) The mitochondrial electron transfer alteration as a factor involved in the aging brain. Neurobiol Aging 12: 361–368
Bridges RJ, Koh J-Y, Hatalski CG, Cotman CW (1991) Increased excitotoxic vulnerability of cortical cultures with reduced levels of glutathione. Eur J Pharmacol 192: 199–200
Chau RMW, Skaper SD, Varon S (1988) Peroxidative block of glucose utilization and survival in CNS neuronal cultures. Neurochem Res 13: 611–616
Damier P, Hirsch EC, Zhang P, Agid Y, Javoy-Agid F (1993) Glutathione peroxidase, glial cells and Parkinson's disease. Neuroscience 52: 1–6
Dexter DT, Carayon A, Javoy-Agid F, Agid Y, Wells FR, Daniel SE, Jenner P, Marsden C (1991) Alterations in the levels of iron, ferritin and other trace metals in Parkinson's disease and other neurogedenerative disease affecting the basla ganglia. Brain 114: 1953–1975
Dexter DT, Sian J, Rose S, Hindmarsh JG, Mann VM, Cooper JM, Wells FR, Danial SE, Lees AJ, Schapira AHV, Jenner P, Marsden CD (1994) Indices of oxidative stress and mitochondrial function in individuals with incidental Lewy body disease. Ann Neurol 35: 38–44
Di Monte DA, Chan P, Sandy MS (1992) Glutathione in Parkinson's disease: a link between oxidative stress and mitochondrial damage? Ann Neurol 32: S111-S115
Fearnley JM, Lees AJ (1991) Aging and Parkinson's disease: substantia nigra regional selectivity. Brain 114: 2283–2301
Forno LS (1969) Concentric hyalin intraneuronal inclusions of Lewy type in the brains of elderly persons (50 incidental cases). Relationship to parkinsonism. J Am Geriatr Soc 17: 557–575
Gibb WRG (1986) Idiopathic Parkinson's disease and the Lewy body disorders. Neuropathol Appl Neurobiol 12: 223–234
Gibb WRG, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson's disease. J Neurol Neurosurg Psychiatry 51: 745–752
Han S-K, Mytilineou C, Cohen G (1996) L-DOPA up-regulates glutathione and protects mesencephalic cultures against oxidative stress. J Neurochem 66: 501–510
Heafield TH, Fearn S, Steventon GB, Waring RH, Williams AC, Sturman SG (1990) Plasma cysteine and sulphate levels in patients with motor neurone, Parkinson's and Alzheimer's disease. Neurosci Lett 110: 216–220
Hjelle OP, Chaudhry FA, Ottersen OP (1994) Antisera to glutathione: characterization and immunocytochemical application to the rat cerebellum. Eur J Neurosci 6: 793–804
Huang J, Philbert MA (1995) Distribution of glutathione and glutathione-related enzyme systems in mitochondria and cytosol of cultured cerebellar astrocytes and granule cells. Brain Res 680: 16–22
Jain A, Martensson J, Stole E, Auld PAM, Meister A (1991) Glutathione deficiency leads to mitochondrial damage in brain. Proc Natl Acad Sci USA 88: 1913–1917
Jenner P, Dexter DT, Sian J, Schapira AHV, Marsden CD (1992) Oxidative stress as a cause of nigral cell death in Parkinson's disease and incidental Lewy body disease. Ann Neurol 32: 582–587
Jenner P, Schapira AHV, Marsden CD (1992) New insights into the cause of Parkinson's disease. Neurology 42: 2241–2250
Kirstein CL, Coopersmith R, Bridges RJ, Leon M (1991) Glutathione levels in olfactory and non-olfactory neural structures of rats. Brain Res 543: 341–346
Makar TK, Nefergaard M, Preuss A, Gelbard AS, Perumal AS, Cooper AJL (1994) Vitamin E, ascorbate, glutathione, glutathione disulfide, and enzymes of glutathione metabolism in cultures of chick astrocytes and neurons: evidence that astrocytes play an important role in antioxidative processes in the brain. J Neurochem 62: 45–53
Marttila RJ, Lorentz H, Rinne UK (1988) Oxygen toxicity protecting enzymes in Parkinson's disease: increase of superoxide dismutase-like activity in the substantia nigra and basal nucleus. J Neurol Sci 86: 321–331
McGeer PL, Itagaki S, Akiyama H, McGeer EG (1988) Rate of cell death in parkinsonism indicates an active neuropathological process. Ann Neurol 24: 574–576
McGeer PL, Kawamata T, Douglas GW, et al (1993) Microglia in degenerative neurological disease. Glia 7: 84–92
Meister A (1992) On the antioxidant effects of ascorbic acid and glutathione. Biochem Pharmacol 44: 1905–1915
Meister A (1995) Mitochondrial changes associated with gluthatione deficiency. Biochim Biophys Acta 1271: 35–42
Perry TL, Yong VW (1986) Idiopathic Parkinson's disease, progressive supranuclear palsy and glutathione metabolism in the substantia nigra of patients. Neurosci Lett 67: 269–274
Perry TL, Godwin DV, Hansen S (1982) Parkinson's disease: a disorder due to nigral glutathione deficiency. Neurosci Lett 33: 305–310
Philbert MA, Beiswanger CD, Waters DK, Reuhl KR, Lowndes HE (1991) Cellular and regional distribution of reduced glutathione in the nervous system of the rat: histochemical localisation by mercury orange and O-phthaldialdiadehyde-induced histofluorescence. Toxicol Appl Pharmacol 107: 215–227
Pileblad E, Fornstedt B, Magnusson T (1989) Reduction of brain glutathione by L-buthione sulfoximine potentiates the dopamine-depleting action of 6-hydroxydopamine in rat striatum. J Neurochem 52: 978–980
Pileblad E, Erikksson PS, Hansson E (1991) The presence of glutathione in primary neuronal and astroglial cultures from rat cerebral cortex and brain stem. J Neural Transm 86: 43–49
Raps SP, Lai JCK, Hertz L, Cooper AJL (1989) Glutathione is present in high concentrations in cultured astrocytes but not in cultured neurons. Brain Res 493: 398–401
Riederer P, Sofic E, Rausch W-D, Schmidt B, Reynolds GB, Jellinger K, Youdim MBH (1989) Transition metals, ferritin, glutathione and ascorbic acid in parkinsonian brains. J Neurochem 52: 515–520
Sagara J, Miura K, Bannai S (1993) Maintenance of neuronal glutathione by glial cells. J Neurochem 61: 1672–1676
Saggu H, Cooksey J, Dexter D, Wells FR, Lees A, Jenner P, Marsden CD (1989) A selective increase in particulate superoxide dismutase activity in parkinsonian substantia nigra. J Neurochem 53: 692–697
Schapira AHV, Mann VM, Cooper JM, Dexter D, Daniel SE, Jenner P, Clark JB, Marsden CD (1990) The anatomic and disease specificity of NADH CoQ reductase (complex I) deficiency in Parkinson's disease. J Neurochem 55: 2142–2145
Sian J, Dexter DT, Lees AJ, Daniel S, Agid Y, Javoy-Agid F, Jenner P, Marsden CD (1994) Alterations in glutathione levels in Parkinson's disease and other neurodegenerative disorders affecting the basal ganglia. Ann Neurol 36: 348–355
Sian J, Dexter DT, Lees AJ, Jenner P, Marsden CD (1994) Glutathione-related enzymes in brain in Parkinson's disease. Ann Neurol 36: 356–361
Slivka A, Mytilineou C, Cohen G (1987) Histochemical evaluation of gluthathione in brain. Brain Res 409: 275–284
Wefers H, Sies H (1988) The protection by ascorbate and glutathione against microsomal lipid peroxidation is dependent on vitamin E. Eur J Biochem 174: 353–357
Zängerle L, Cuénod M, Winterhalter KH, Do KQ (1992) Screening of thiol compounds: depolarization-induced release of glutathione and cysteine from rat brain slices. J Neurochem 59: 181–189
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Pearce, R.K.B., Owen, A., Daniel, S. et al. Alterations in the distribution of glutathione in the substantia nigra in Parkinson's disease. J. Neural Transmission 104, 661–677 (1997). https://doi.org/10.1007/BF01291884
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF01291884