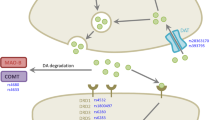
Abstract
Levodopa-induced dyskinesia (LID) is an adverse effect that negatively impacts the quality of life of patients with Parkinson’s disease (PD). Studies report that genetic variations in the genes of the pharmacogenetic pathway of the levodopa (L-DOPA) might be associated with LID development. The goal of the present study was to investigate a possible influence of functional genetic variants in the DRD1 (rs4532), DRD2 (rs1800497), DAT1 (rs28363170), and COMT (rs4680) genes with LID development. A total of 220 patients with idiopathic PD were enrolled. The genotyping for DRD1 (rs4532), DRD2 (rs1800497), DAT1 (rs28363170), and COMT (rs4680) polymorphisms were performed using Restriction Fragment Length Polymorphism (PCR–RFLP). Univariate and multivariate analyses were performed to assess the association of these polymorphisms and risk factors with LID development. Multivariate Cox regression analysis showed increased risk to LID development for both Levodopa Dose Equivalency (LED) (Hazard ratios (HR) = 1.001; 95% CI 1.00–1.01; p = 0.009) and individuals carrying the COMT L/L genotype (HR = 2.974; 95% CI 1.12–7.83; p = 0.010). Furthermore, when performed a Cox regression analysis adjusted for a total LED, we observed that the genotype COMT L/L had a 3.84–fold increased risk for LID development (HR = 3.841; 95% CI 1.29–11.37; p = 0.012). Our results suggest that before treating LID in PD patients, it is important to take into consideration genetic variant in the COMT gene, since COMT LL genotype may increase the risk for LID development.

Similar content being viewed by others
References
Kalia LV, Lang AE (2015) Parkinson’s disease. Lancet 386(9996):896–912. https://doi.org/10.1016/s0140-6736(14)61393-3
Poewe W et al (2017) Parkinson disease. Nat Rev Dis Primer 3:17013. https://doi.org/10.1038/nrdp.2017.13
Sauerbier A et al (2018) Impact of ethnicity on the natural history of Parkinson disease. Med J Aust 208(9):410–414. https://doi.org/10.5694/mja17.01074
Dorsey ER, Bloem BR (2018) The Parkinson pandemic: a call to action. JAMA Neurol 75(1):9. https://doi.org/10.1001/jamaneurol.2017.3299
Hess C, Hallett M (2017) The phenomenology of Parkinson’s disease. Semin Neurol 37(02):109–117. https://doi.org/10.1055/s-0037-1601869
Dickson DW (2018) Neuropathology of Parkinson disease. Parkinsonism Relat Disord 46:S30–S33. https://doi.org/10.1016/j.parkreldis.2017.07.033
Zeng X-S et al (2018) Cellular and molecular basis of neurodegeneration in Parkinson disease. Front Aging Neurosci. https://doi.org/10.3389/fnagi.2018.00109
Olanow CW (2019) Levodopa is the best symptomatic therapy for PD: nothing more, nothing less. Mov Disord. https://doi.org/10.1002/mds.27690
Tran TN et al (2018) Levodopa-induced dyskinesia: clinical features, incidence, and risk factors. J Neural Transm 125(8):1109–1117. https://doi.org/10.1007/s00702-018-1900-6
Verschuur CVM et al (2019) Randomized delayed-start trial of levodopa in Parkinson’s disease. N Engl J Med 380(4):315–324. https://doi.org/10.1056/nejmoa1809983
You H et al (2018) Molecular basis of dopamine replacement therapy and its side effects in Parkinson’s disease. Cell Tissue Res 373(1):111–135. https://doi.org/10.1007/s00441-018-2813-2
Alice M et al (2012) Levodopa-induced-dyskinesias clinical features, incidence, risk factors, management and impact on quality of life. J Park Dis 3:189–198. https://doi.org/10.3233/jpd-2012-120103
Eusebi P et al (2018) Risk factors of levodopa-induced dyskinesia in Parkinson’s disease: results from the PPMI cohort. Npj Park Dis. https://doi.org/10.1038/s41531-018-0069-x
Ray Chaudhuri K et al (2018) Motor and nonmotor complications of levodopa: phenomenology, risk factors, and imaging features: l-dopa motor and nonmotor complications in PD. Mov Disord 33(6):909–919. https://doi.org/10.1002/mds.27386
Sharma JC et al (2010) Classifying risk factors for dyskinesia in Parkinson’s disease. Parkinsonism Relat Disord 16(8):490–497. https://doi.org/10.1016/j.parkreldis.2010.06.003
Turcano P et al (2018) Levodopa-induced dyskinesia in Parkinson disease: a population-based cohort study. Neurology 91(24):e2238–e2243. https://doi.org/10.1212/wnl.0000000000006643
Espay AJ et al (2018) Levodopa-induced dyskinesia in Parkinson disease: current and evolving concepts: dyskinesia in PD. Ann Neurol 84(6):797–811. https://doi.org/10.1002/ana.25364
Leta V et al (2019) Can therapeutic strategies prevent and manage dyskinesia in Parkinson’s disease? An update. Expert Opin Drug Saf 18(12):1203–1218. https://doi.org/10.1080/14740338.2019.1681966
Ciccacci C, Borgiani P (2019) Pharmacogenomics in Parkinson’s disease: which perspective for developing a personalized medicine? Neural Regen Res 14(1):75. https://doi.org/10.4103/1673-5374.243706
dos Santos EUD et al (2018) The influence of SLC6A3 and DRD2 polymorphisms on levodopa-therapy in patients with sporadic Parkinson’s disease. J Pharm Pharmacol. https://doi.org/10.1111/jphp.13031
Kalinderi K et al (2019) Pharmacogenetics and levodopa induced motor complications. Int J Neurosci 129(4):384–392. https://doi.org/10.1080/00207454.2018.1538993
Sampaio TF et al (2018) MAO-B and COMT genetic variations associated with levodopa treatment response in patients with Parkinson’s disease. J Clin Pharmacol. https://doi.org/10.1002/jcph.1096
Tomlinson CL et al (2010) Systematic review of levodopa dose equivalency reporting in Parkinson’s disease: systematic review of LED reporting in PD. Mov Disord 25(15):2649–2653. https://doi.org/10.1002/mds.23429
Comi C et al (2017) Polymorphisms of dopamine receptor genes and risk of L-dopa-induced dyskinesia in Parkinson’s disease. Int J Mol Sci 18(2):242. https://doi.org/10.3390/ijms18020242
Freitas M et al (2017) Motor complications of dopaminergic medications in Parkinson’s disease. Semin Neurol 37(02):147–157. https://doi.org/10.1055/s-0037-1602423
Kaiser R et al (2003) L -dopa-induced adverse effects in PD and dopamine transporter gene polymorphism. Neurology 60(11):1750–1755
Politi C et al (2018) Genetics and treatment response in Parkinson’s disease: an update on pharmacogenetic studies. NeuroMol Med 20(1):1–17. https://doi.org/10.1007/s12017-017-8473-7
Schumacher-Schuh AF et al (2014) Parkinson’s disease pharmacogenomics: new findings and perspectives. Pharmacogenomics 15(9):1253–1271. https://doi.org/10.2217/pgs.14.93
Strafella C et al (2018) Application of precision medicine in neurodegenerative diseases. Front Neurol. https://doi.org/10.3389/fneur.2018.00701
dos Santos EUD et al (2019) Influence of DRD1 and DRD3 polymorphisms in the occurrence of motor effects in patients with sporadic Parkinson’s disease. NeuroMol Med. https://doi.org/10.1007/s12017-019-08549-3
Bialecka M et al (2008) The association of functional catechol-O-methyltransferase haplotypes with risk of Parkinsonʼs disease, levodopa treatment response, and complications. Pharmacogenet Genom 18(9):815–821. https://doi.org/10.1097/fpc.0b013e328306c2f2
de Lau LML et al (2012) Catechol-O-methyltransferase Val158Met and the risk of dyskinesias in Parkinson’s disease. Mov Disord 27(1):132–135. https://doi.org/10.1002/mds.23805
Rieck M et al (2012) DRD2 haplotype is associated with dyskinesia induced by levodopa therapy in Parkinson’s disease patients. Pharmacogenomics 13(15):1701–1710. https://doi.org/10.2217/pgs.12.149
Kaplan N et al (2014) Sequence variants in SLC6A3, DRD2, and BDNF genes and time to levodopa-induced dyskinesias in Parkinson’s disease. J Mol Neurosci 53(2):183–188. https://doi.org/10.1007/s12031-014-0276-9
Wu H et al (2014) Catechol-O-methyltransferase Val158Met polymorphism: modulation of wearing-off susceptibility in a Chinese cohort of Parkinson’s disease. Parkinsonism Relat Disord 20(10):1094–1096. https://doi.org/10.1016/j.parkreldis.2014.07.011
Hutz MH, Rieder CR (2018) The future of pharmacogenetics in Parkinson’s disease treatment. Pharmacogenomics 19(3):171–174. https://doi.org/10.2217/pgs-2017-0180
Payami H (2017) The emerging science of precision medicine and pharmacogenomics for Parkinson’s disease: precision medicine and pharmacogenomics of PD. Mov Disord 32(8):1139–1146. https://doi.org/10.1002/mds.27099
Redenšek S et al (2019) Dopaminergic pathway genes influence adverse events related to dopaminergic treatment in Parkinson’s disease. Front Pharmacol. https://doi.org/10.3389/fphar.2019.00008
Titova N, Chaudhuri KR (2017) Personalized medicine in Parkinson’s disease: time to be precise: Parkinson’s and personalized medicine. Mov Disord 32(8):1147–1154. https://doi.org/10.1002/mds.27027
Hughes AJ et al (1992) What features improve the accuracy of clinical diagnosis in Parkinson’s disease: a clinicopathologic study. Neurology 42(6):1142–1146
Martínez-Martín P et al (1994) Unified Parkinson’s disease rating scale characteristics and structure: UNIFIED PARKINSON’S DISEASE RATING SCALE. Mov Disord 9(1):76–83. https://doi.org/10.1002/mds.870090112
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17(5):427–442
Damasceno dos Santos EU et al (2019) Pharmacogenetic profile and the occurrence of visual hallucinations in patients with sporadic Parkinson’s disease. J Clin Pharmacol. https://doi.org/10.1002/jcph.1394
Balestrino R, Martinez-Martin P (2017) Neuropsychiatric symptoms, behavioural disorders, and quality of life in Parkinson’s disease. J Neurol Sci 373:173–178. https://doi.org/10.1016/j.jns.2016.12.060
Bovolenta T et al (2017) Average annual cost of Parkinson’s disease in São Paulo, Brazil, with a focus on disease-related motor symptoms. Clin Interv Aging 12:2095–2108. https://doi.org/10.2147/cia.s151919
Rodríguez Vicente AE et al (2018) Personalized medicine into health national services: barriers and potentialities. Drug Metab Pers Ther 33(4):159–163. https://doi.org/10.1515/dmpt-2018-0017
Watanabe M et al (2003) Association between Catechol-O-methyltransferase gene polymorphisms and wearing-off and dyskinesia in Parkinson’s disease. Neuropsychobiology 48(4):190–193. https://doi.org/10.1159/000074637
Muellner J et al (2015) Dopaminergic denervation severity depends on COMT Val158Met polymorphism in Parkinson’s disease. Parkinsonism Relat Disord 21(5):471–476. https://doi.org/10.1016/j.parkreldis.2015.02.009
Moreau C et al (2015) Polymorphism of the dopamine transporter type 1 gene modifies the treatment response in Parkinson’s disease. Brain 138(5):1271–1283. https://doi.org/10.1093/brain/awv063
Fuke S et al (2001) The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J 1(2):152–156
Acknowledgement
We thank the patients and their families, whose collaboration and understanding have made this work possible.
Funding
This study was supported by the Brazilian funding agency FACEPE (Fundação de Amparo à Ciência e Tecnologia do Estado de Pernambuco) and in part by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior—Brasil (CAPES)—Finance Code 001.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest and no competing financial interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
dos Santos, E.U.D., da Silva, I.I.F.G., Asano, A.G.C. et al. Pharmacogenetic profile and the development of the dyskinesia induced by levodopa-therapy in Parkinson’s disease patients: a population-based cohort study. Mol Biol Rep 47, 8997–9004 (2020). https://doi.org/10.1007/s11033-020-05956-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11033-020-05956-9