Abstract
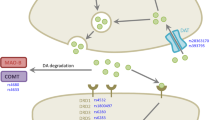
Levodopa-induced dyskinesias (LID) present a common but elusive complication of levodopa therapy in Parkinson’s disease (PD). In order to identify genetic factors associated with LID, 352 (213 males) levodopa-treated Israeli PD patients were genotyped for 34 polymorphisms within three candidate genes affecting dopaminergic activity and synaptic plasticity: dopamine transporter gene (DAT1 or SLC6A3) [14 single nucleotide polymorphisms (SNPs) and 40-bp variable number tandem repeat (VNTR)], DRD2 [11 SNPs and dinucleotide CA short tandem repeat (STR)], and BDNF (7 SNPs). A comparison of patients with and without LID was performed by applying a time-oriented approach, with survival analyses evaluating LID development hazard rate over time [Cox proportional hazards and accelerated failure time (AFT) lognormal models]. Overall, 192 (54.5 %) participants developed LID, with a mean latency of 5.0 (±4.5) years. After adjusting for gender, age at PD onset, duration of symptoms prior to levodopa exposure, and multiple testing correction, one SNP in SLC6A3 (with 81 % genotyping success) was significantly associated with LID latency: the C allele of the rs393795 extended the time to LID onset, time ratio = 4.96 (95 % CI, 2.3–10.9; p = 4.1 × 10−5). This finding should be validated in larger, ethnically diverse PD populations, and the biological mechanism should be explored.

Similar content being viewed by others
References
Bezard E, Brotchie JM, Gross CE (2001) Pathophysiology of levodopa-induced dyskinesia: potential for new therapies. Nat Rev Neurosci 2(8):577–588
Bialecka M, Drozdzik M, Klodowska-Duda G et al (2004) The effect of monoamine oxidase B (MAOB) and catechol-O-methyltransferase (COMT) polymorphisms on levodopa therapy in patients with sporadic Parkinson’s disease. Acta Neurol Scand 110(4):260–266
Cartegni L, Chew SL, Krainer AR (2002) Listening to silence and understanding nonsense: exonic mutations that affect splicing. Nat Rev Genet 3(4):285–298
Cummins TDR, Hawi Z, Hocking J (2012) Dopamine transporter genotype predicts behavioural and neural measures of response inhibition. Mol Psychiatry 17(11):1086–1092
de la Fuente-Fernández R, Sossi V, Huang Z et al (2004) Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson’s disease: implications for dyskinesias. Brain 127(Pt 12):2747–2754
Egan MF, Kojima M, Callicott JH et al (2003) The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell 112(2):257–269
Foltynie T, Cheeran B, Williams-Gray CH et al (2009) BDNF val66met influences time to onset of levodopa induced dyskinesia in Parkinson’s disease. J Neurol Neurosurg Psychiatry 80(2):141–144
Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S (2001) The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J 1(2):152–156
Hassin-Baer S, Molchadski I, Cohen OS et al (2011) Gender effect on time to levodopa-induced dyskinesias. J Neurol 258(11):2048–2053
Hauser RA, McDermott MP, Messing S (2006) Factors associated with the development of motor fluctuations and dyskinesias in Parkinson disease. Arch Neurol 63(12):1756–1760
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry 55(3):181–184
Jankovic J (2005) Motor fluctuations and dyskinesias in Parkinson’s disease: clinical manifestations. Mov Disord 20(Suppl 11):S11–S16
Kaiser R, Hofer A, Grapengiesser A et al (2003) L-Dopa-induced adverse effects in PD and dopamine transporter gene polymorphism. Neurology 60(11):1750–1755
Khor SP, Hsu A (2007) The pharmacokinetics and pharmacodynamics of levodopa in the treatment of Parkinson’s disease. Curr Clin Pharmacol 2(3):234–243
Korbie DJ, Mattick JS (2008) Touchdown PCR for increased specificity and sensitivity in PCR amplification. Nat Protoc 3(9):1452–1456
Korczyn AD (2011) Is there a need to redefine Parkinson’s disease? J Neurol Sci 310(1–2):2–3
Kurian MA, Li Y, Zhen J et al (2011) Clinical and molecular characterisation of hereditary dopamine transporter deficiency syndrome: an observational cohort and experimental study. Lancet Neurol 10(1):54–62
Lee JY, Cho J, Lee EK, Park SS, Jeon BS (2011) Differential genetic susceptibility in diphasic and peak-dose dyskinesias in Parkinson’s disease. Mov Disord 26(1):73–79
Molchadski I, Korczyn AD, Cohen OS et al (2011) The role of apolipoprotein E polymorphisms in levodopa-induced dyskinesia. Acta Neurol Scand 123(2):117–121
Morgante F, Espay AJ, Gunraj C, Lang AE, Chen R (2006) Motor cortex plasticity in Parkinson’s disease and levodopa-induced dyskinesias. Brain 129(Pt 4):1059–1069
Muenter MD, Tyce GM (1971) L-Dopa therapy of Parkinson’s disease: plasma L-dopa concentration, therapeutic response, and side effects. Mayo Clin Proc 46(4):231–239
Nutt JG (2001) Motor fluctuations and dyskinesia in Parkinson’s disease. Parkinsonism Relat Disord 8(2):101–108
Oeth P, Beaulieu M, Park C et al (2005) iPLEX™ assay: increased plexing efficiency and flexibility for MassARRAY® system through single base primer extension with mass-modified terminators [Sequenom application note]. Sequenom, San Diego
Oliveri RL, Annesi G, Zappia M et al (1999) Dopamine D2 receptor gene polymorphism and the risk of levodopa-induced dyskinesias in PD. Neurology 53(7):1425–1430
Rascol O, Brooks DJ, Korczyn AD, De Deyn PP, Clarke CE, Lang AE (2000) A five-year study of the incidence of dyskinesia in patients with early Parkinson’s disease who were treated with ropinirole or levodopa. 056 Study group. N Engl J Med 342(20):1484–1491
Sossi V, de la Fuente-Fernández R, Schulzer M, Troiano AR, Ruth TJ, Stoessl AJ (2007) Dopamine transporter relation to dopamine turnover in Parkinson’s disease: a positron emission tomography study. Ann Neurol 62(5):468–474
Strong JA, Dalvi A, Revilla FJ et al (2006) Genotype and smoking history affect risk of levodopa-induced dyskinesias in Parkinson’s disease. Mov Disord 21(5):654–659
Talkowski ME, McCann KL, Chen M et al (2010) Fine-mapping reveals novel alternative splicing of the dopamine transporter. Am J Med Genet B Neuropsychiatr Genet 153B(8):1434–1447
Uhl GR (2003) Dopamine transporter: basic science and human variation of a key molecule for dopaminergic function, locomotion, and Parkinsonism. Mov Disord 18(Suppl 7):S71–S80
van Munster BC, Yazdanpanah M, Tanck MW et al (2010) Genetic polymorphisms in the DRD2, DRD3, and SLC6A3 gene in elderly patients with delirium. Am J Med Genet B Neuropsychiatr Genet 153B(1):38–45
Wei LJ (1992) The accelerated failure time model: a useful alternative to the Cox regression model in survival analysis. Stat Med 11(14–15):1871–1879
Yahalom G, Kaplan N, Vituri A et al (2012) Dyskinesias in patients with Parkinson’s disease: effect of the leucine-rich repeat kinase 2 (LRRK2) G2019S mutation. Parkinsonism Relat Disord 18(9):1039–1041
Acknowledgments
Special appreciation is due to Ms. Julia Feiler and Prof. Gideon Rechavi’s research laboratories, at Sheba Cancer Research Center laboratories, for the use of their facilities and equipment and assistance with operating the Sequenom MassARRAY® System technology. This study was supported by the Bharier Medical Fund, in memory of Nat and Sophie Bharier, and by the Mariana and George Saia Foundation (Tel Aviv University).
Conflict of interest
The authors have no conflicts of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Additional information
Conflict of Interest
The authors have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Kaplan, N., Vituri, A., Korczyn, A.D. et al. Sequence Variants in SLC6A3, DRD2, and BDNF Genes and Time to Levodopa-Induced Dyskinesias in Parkinson’s Disease. J Mol Neurosci 53, 183–188 (2014). https://doi.org/10.1007/s12031-014-0276-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12031-014-0276-9