Abstract
Background
Dopamine replacement therapy remains the gold standard for symptomatic management of Parkinson’s disease worldwide. However, most patients will develop debilitating motor levodopa-induced complications (MLIC) in the form of levodopa-induced dyskinesia (LID) and/or motor fluctuations (MF). This study aimed to conduct a systematic review and meta-analysis on the pharmacogenetic association between LID and MF with common genetic variants of the dopamine metabolic and signaling pathways.
Methods
A meta-analysis was conducted according to the PRISMA guidelines. Extracted studies include case–control studies evaluating the association between SLC6A3/DAT rs28363170 and rs393795; COMT rs4680 and rs4633; MAO-B rs1799836, BDNF rs6265, DRD1 rs4532, DRD2 rs1800497, DRD3 rs6280, and DRD5 rs6283 polymorphisms; and the overall risk of MLIC and its subtypes LID or MF. Genotypic frequency were tested for deviation from the Hardy–Weinberg equilibrium (HWE), and the genetic association was examined using the allelic (a vs. A), recessive (aa vs. Aa + AA), dominant (aa + Aa vs. AA), overdominant (Aa vs. aa + AA), homozygous (aa vs. AA), and heterozygous (Aa vs. AA and aa vs. aA) models.
Results
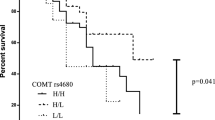
Fourteen studies were included in the meta-analysis. A significant association was found between COMT rs46809 polymorphisms with LID but not MF, with the association observable in Asians but not Caucasians. In Asians, the COMT rs4633 was significantly associated with the occurrence of both LID and MF. The MAO-B rs1799836 was associated with both MF and LID. Among all the dopamine receptor genes analyzed, only DRD2 exhibited an association with LID. No association was observed between the SLC6AT/DAT and BDNF genes with either LID or MF.
Conclusion
Strong associations were observed between polymorphisms of genes regulating dopamine metabolism with the occurrence of LID and/or MF. The MAO-B rs1799836 may be potential for use as a general pharmacogenetic marker of MLIC, while the COMT rs4680 and rs4633 may be used as markers of LID in Asian ethnicities.





Similar content being viewed by others
Abbreviations
- BDNF:
-
Brain-derived neurotrophic factor
- CI:
-
Confidence interval
- COMT:
-
Catechol-O-methyltransferase
- DAT:
-
Dopamine transporter
- DRD:
-
Dopamine receptor
- HWE:
-
Hardy-Weinberg equilibrium
- L-Dopa:
-
L-dopamine
- LID:
-
Levodopa-induced dyskinesia
- MAO:
-
Monoamine oxidase
- MF:
-
Motor fluctuations
- MLIC:
-
Motor levodopa-induced complications
- OR:
-
Odds ratio
- PD:
-
Parkinson’s disease
- PRISMA:
-
Preferred Reporting Items for Systematic Reviews and Meta-Analysis
- SLC6:
-
Solute carrier 6
- SNP:
-
Single nucleotide polymorphism
References
Poewe W, Seppi K, Tanner CM, Halliday GM, Brundin P, Volkmann J et al (2017) Parkinson disease. Nat Rev Dis Primers 3(1):1–21
Dorsey ER, Elbaz A, Nichols E, Abd-Allah F, Abdelalim A, Adsuar JC et al (2018) Global, regional, and national burden of Parkinson’s disease, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17(11):939–953
Moore DJ, West AB, Dawson VL, Dawson TM (2005) Molecular pathophysiology of Parkinson’s disease. Annu Rev Neurosci 28(1):57–87
Schulz-Schaeffer WJ (2010) The synaptic pathology of α-synuclein aggregation in dementia with Lewy bodies, Parkinson’s disease and Parkinson’s disease dementia. Acta Neuropathol 120(2):131–143
Bergman H, Deuschl G (2002) Pathophysiology of Parkinson’s disease: from clinical neurology to basic neuroscience and back. Mov Disord 17(S3):S28-40
Lindenbach D, Bishop C (2013) Critical involvement of the motor cortex in the pathophysiology and treatment of Parkinson’s disease. Neurosci Biobehav Rev 37(10):2737–2750
Poewe W, Antonini A, Zijlmans JC, Burkhard PR, Vingerhoets F (2010) Levodopa in the treatment of Parkinson’s disease: an old drug still going strong. Clin Interv Aging 5:229
Ahlskog JE, Muenter MD (2001) Frequency of levodopa-related dyskinesias and motor fluctuations as estimated from the cumulative literature. Mov Disord 16(3):448–458
Kalinderi K, Papaliagkas V, Fidani L (2019) Pharmacogenetics and levodopa induced motor complications. Int J Neurosci 129(4):384–392
Kalinderi K, Fidani L, Katsarou Z, Bostantjopoulou S (2011) Pharmacological treatment and the prospect of pharmacogenetics in Parkinson’s disease. Int J Clin Pract 65(12):1289–1294
Hisahara S, Shimohama S (2011) Dopamine receptors and Parkinson’s disease. Int J Med Chem: 403039
Hyman C, Hofer M, Barde Y-A, Juhasz M, Yancopoulos GD, Squinto SP et al (1991) BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature 350(6315):230–232
Falla M, Di Fonzo A, Hicks AA, Pramstaller PP, Fabbrini G (2021) Genetic variants in levodopa-induced dyskinesia (LID): a systematic review and meta-analysis. Parkinsonism Relat Disord 84:52–60
Moher D, Liberati A, Tetzlaff J, Altman DG, Prisma Group (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 6(7):e1000097
Ulhaq ZS, Soraya GV, Milliana A, Tse WKF (2021) Association between GPER gene polymorphisms and GPER expression levels with cancer predisposition and progression. Heliyon 7(3):e06428
Ulhaq ZS, Soraya GV, Budu Wulandari LR (2020) The role of IL-6–174 G/C polymorphism and intraocular IL-6 levels in the pathogenesis of ocular diseases: a systematic review and meta-analysis. Sci Rep 10(1):1–20
Ulhaq ZS, Soraya GV (2020) Anti-IL-6 receptor antibody treatment for severe COVID-19 and the potential implication of IL-6 gene polymorphisms in novel coronavirus pneumonia. Med Clin 155(12):548
Ulhaq Z, Soraya G (2020) Aqueous humor interleukin-6 levels in primary open-angle glaucoma (POAG): a systematic review and meta-analysis. Arch Soc Esp Oftalmol (Engl Ed) 95(7):315–321
Soraya GV, Ulhaq ZS (2020) Crucial laboratory parameters in COVID-19 diagnosis and prognosis: an updated meta-analysis. Med Clin (Barc) 155(4):143–151
Ulhaq Z (2020) Chemokine IL-8 level in aqueous humor of open-angle glaucoma: a meta-analysis. Arch Soc Esp Oftalmol (Engl Ed) 95(3):114–119
Purcaro C, Vanacore N, Moret F, Di Battista ME, Rubino A, Pierandrei S et al (2019) DAT gene polymorphisms (rs28363170, rs393795) and levodopa-induced dyskinesias in Parkinson’s disease. Neurosci Lett 690:83–88
Kakinuma S, Beppu M, Sawai S, Nakayama A, Hirano S, Yamanaka Y et al (2020) Monoamine oxidase B rs1799836 G allele polymorphism is a risk factor for early development of levodopa-induced dyskinesia in Parkinson’s disease. eNeurologicalSci 19:100239
Dos Santos EUD, Sampaio TF, Tenório dos Santos AD, Bezerra Leite FC, da Silva RC, Crovella S et al (2019) The influence of SLC 6A3 and DRD 2 polymorphisms on levodopa-therapy in patients with sporadic Parkinson’s disease. J Pharm Pharmacol 71(2):206–12
Michałowska M, Chalimoniuk M, Jówko E, Przybylska I, Langfort J, Toczylowska B et al (2020) Gene polymorphisms and motor levodopa-induced complications in Parkinson’s disease. Brain Behav 10(3):e01537
Sampaio TF, Dos Santos EUD, de Lima GDC, Dos Anjos RSG, da Silva RC, Asano AGC et al (2018) MAO-B and COMT genetic variations associated with levodopa treatment response in patients with Parkinson’s disease. J Clin Pharmacol 58(7):920–926
Watanabe M, Harada S, Nakamura T, Ohkoshi N, Yoshizawa K, Hayashi A et al (2003) Association between catechol-O-methyltransferase gene polymorphisms and wearing-off and dyskinesia in Parkinson’s disease. Neuropsychobiology 48(4):190–193
Cheshire P, Bertram K, Ling H, O’sullivan SS, Halliday G, McLean C et al (2014) Influence of single nucleotide polymorphisms in COMT, MAO-A and BDNF genes on dyskinesias and levodopa use in Parkinson’s disease. Neurodegener Dis 13(1):24–28
Zhao C, Wang Y, Zhang B, Yue Y, Zhang J (2020) Genetic variations in catechol-o-methyltransferase gene are associated with levodopa response variability in Chinese patients with Parkinson’s disease. Sci Rep 10(1):1–8
Hao H, Shao M, An J, Chen C, Feng X, Xie S et al (2014) Association of catechol-O-methyltransferase and monoamine oxidase B gene polymorphisms with motor complications in Parkinson’s disease in a Chinese population. Parkinsonism Relat Disord 20(10):1041–1045
Wu H, Dong F, Wang Y, Xiao Q, Yang Q, Zhao J et al (2014) Catechol-O-methyltransferase Val158Met polymorphism: modulation of wearing-off susceptibility in a Chinese cohort of Parkinson’s disease. Parkinsonism Relat Disord 20(10):1094–1096
Qian Y, Liu J, Xu S, Yang X, Xiao Q (2017) Roles of functional catechol-O-methyltransferase genotypes in Chinese patients with Parkinson’s disease. Transl Neurodegener 6(1):1–11
Comi C, Ferrari M, Marino F, Magistrelli L, Cantello R, Riboldazzi G et al (2017) Polymorphisms of dopamine receptor genes and risk of l-dopa–induced dyskinesia in Parkinson’s disease. Int J Mol Sci 18(2):242
Dos Santos EUD, Duarte EBC, Miranda LMR, Asano AGC, Asano NMJ, Maia M de MD et al (2019) Influence of DRD1 and DRD3 polymorphisms in the occurrence of motor effects in patients with sporadic Parkinson’s disease. Neuromol Med 21(3):295–302
Wang J, Liu Z-L, Chen B (2001) Dopamine D5 receptor gene polymorphism and the risk of levodopa-induced motor fluctuations in patients with Parkinson’s disease. Neurosci Lett 308(1):21–24
Perez-Lloret S, Negre-Pages L, Damier P, Delval A, Derkinderen P, Destée A et al (2017) L-DOPA-induced dyskinesias, motor fluctuations and health-related quality of life: the COPARK survey. Eur J Neurol 24(12):1532–1538
Thakkinstian A, McElduff P, D’Este C, Duffy D, Attia J (2005) A method for meta-analysis of molecular association studies. Stat Med 24(9):1291–1306
Schrag A, Quinn N (2000) Dyskinesias and motor fluctuations in Parkinson’s disease: a community-based study. Brain 123(11):2297–2305
Lechun L, Yu S, Pengling H, Changqi H (2013) The COMT Val158Met polymorphism as an associated risk factor for Parkinson’s disease in Asian rather than Caucasian populations. Neurol India 61(1):12
Kaplan N, Vituri A, Korczyn AD, Cohen OS, Inzelberg R, Yahalom G et al (2014) Sequence variants in SLC6A3, DRD2, and BDNF genes and time to levodopa-induced dyskinesias in Parkinson’s disease. J Mol Neurosci 53(2):183–188
Papapetropoulos S, Argyriou A, Ellul J, Chroni E (2004) Comparison of motor fluctuations and L-dopa-induced dyskinesias in patients with familial and sporadic Parkinson’s disease. Eur J Neurol 11(2):115–119
Nutt JG (2008) Pharmacokinetics and pharmacodynamics of levodopa. Mov Disord 23(S3):S580–S584
Mones R, Elizan T, Siegel G (1971) Analysis of L-dopa induced dyskinesias in 51 patients with parkinsonism. J Neurol Neurosurg Psychiatry 34(6):668–673
Zappia M, Annesi G, Nicoletti G, Arabia G, Annesi F, Messina D et al (2005) Sex differences in clinical and genetic determinants of levodopa peak-dose dyskinesias in Parkinson disease: an exploratory study. Arch Neurol 62(4):601–605
Author information
Authors and Affiliations
Contributions
GS and ZU conceived, designed, and performed the study. GS, ZU, SF, NR, AKB, and MA analyzed and interpreted the data. GS, ZU, SS, MK, SH, MS, AG, and DF aided data collection and extraction. GS and ZU wrote the main draft and revise the manuscript. NR, AKB, and MA critically reviewed the manuscript. All authors reviewed and finally approved the manuscript.
Corresponding author
Ethics declarations
Ethical approval and informed consent
None.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gita Vita Soraya and Zulvikar Syambani Ulhaq have contributed equally to this work and share first authorship.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Soraya, G.V., Ulhaq, Z.S., Shodry, S. et al. Polymorphisms of the dopamine metabolic and signaling pathways are associated with susceptibility to motor levodopa-induced complications (MLIC) in Parkinson’s disease: a systematic review and meta-analysis. Neurol Sci 43, 3649–3670 (2022). https://doi.org/10.1007/s10072-021-05829-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10072-021-05829-4