Abstract
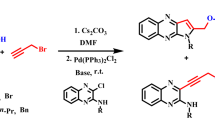
The reactions of several 2-chloroquinoline-3-carboxylate esters with propargyl alcohol and a secondary amine in the presence of palladium catalyst leads to the formation of new alkyl 1-amino substituted pyrrolo[1,2-a]quinoline-4-carboxylate derivatives. This one-pot process, carried out in the absence of any copper salt, provides an efficient method for the synthesis of functionalized pyrrolo[1,2-a]quinolines in good-to-high yields.
Graphical Abstract





Similar content being viewed by others
References
Sonogashira K, Tohda Y, Hagihara N (1975) Convenient synthesis of acetylenes-catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines. Tetrahedron Lett 16:4467–4470. doi:10.1016/S0040-4039(00)91094-3
Cassar L (1975) Synthesis of aryl- and vinyl-substituted acetylene derivatives by the use of nickel and palladium complexes. J Organomet Chem 93:253–257. doi:10.1016/S0022-328X(00)94048-8
Li J, Gribble GW (2000) Palladium in heterocyclic chemistry; tetrahedron organic chemistry series, vol 20. Pergamon, Amsterdam
Willy B, Muller T (2010) Three-component synthesis of benzo[b][1,5]thiazepines via coupling-addition-cyclocondensation sequence. Mol Divers 14:443–453. doi:10.1007/s11030-009-9223-z
Diederich F, Stang P, Tykwinsk R (2005) Acetylene chemistry: chemistry, biology, and material science. Wiley-VCH, Weinheim. doi:10.1002/3527605487
Francke V, Mangel T, Müllen K (1998) Synthesis of \(\alpha \),\(\omega \)-difunctionalized oligo- and poly(p-phenyleneethynylene)s. Macromolecules 31:2447–2453. doi:10.1021/ma971429m
Sonogashira K (2002) Development of Pd-Cu catalyzed cross-coupling of terminal acetylenes with sp\(^2\)-carbon halides. J Organomet Chem 653:46–49. doi:10.1016/S0022-328X(02)01158-0
Vasconcelos SN, Shamim A, Ali B, Oliveira IM, Stefani HA (2016) Functionalization of protected tyrosine via Sonogashira reaction. Mol Divers 20:469–481. doi:10.1007/s11030-015-9642-y
Arumugasamy E, Yu-Hsiang W, Tong-Ing H (2003) Sonogashira coupling reaction with diminished homocoupling. Org Lett 5:1841–1844. doi:10.1021/ol034320+
Siemsen P, Livingston RC, Diederich F (2000) Acetylenic coupling: a powerful tool in molecular construction. Angew Chem Int Ed 39:2632–2657. doi:10.1002/1521-3773(20000804)39:15<2632::AID-ANIE2632>3.0.CO;2-F
Cheng J, Sun Y, Wang F, Guo M, Xu J, Pan Y, Zhang Z (2004) A Copper- and amine-free sonogashira reaction employing aminophosphines as ligands. J Org Chem 69:5428–5432. doi:10.1021/jo049379o
Zhong H, Wang J, Lia L, Wang R (2014) The copper-free Sonogashira cross-coupling reaction promoted by palladium complexes of nitrogen-containing chelating ligands in neat water at room temperature. Dalton Trans 43:2098–2103. doi:10.1039/C3DT52970C
Leadbeater NE, Tominack BJ (2003) Rapid, easy copper-free Sonogashira couplings. Tetrahedron Lett 44:8653–8656. doi:10.1016/j.tetlet.2003.09.159
Böhm V, Herrmann WA (2000) A copper-free procedure for the palladium-catalyzed Sonogashira reaction. Eur J Org Chem 2000:3679–3681. doi:10.1002/1099-0690(200011)2000:22<3679::AID-EJOC3679>3.0.CO;2-X
Anderson KW, Buchwald SL (2005) General catalysts for the Suzuki–Miyaura and Sonogashira coupling reactions of aryl chlorides and for the coupling of challenging substrate combinations in water. Angew Chem Int Ed 44:6173–6177. doi:10.1002/anie.200502017
Yi Ch, Hua R (2006) Efficient copper-free \(\text{ PdCl }_{2}\)(PCy\(_{3}\))\(_{2}\)-catalyzed Sonogashira coupling of aryl chlorides with terminal alkynes. J Org Chem 71:2535–2537. doi:10.1021/jo0525175
Zhang Z, Lu W, Huang W, Li Y, Gao H, Luo Y (2006) Copper-free Sonogashira reaction using 7-chloro camptothecins. Tetrahedron 62:2465–2470. doi:10.1016/j.tet.2006.01.001
Fleckenstein CA, Plenio H (2008) Aqueous/organic cross coupling: sustainable protocol for Sonogashira reactions of heterocycles. Green Chem 10:563–570. doi:10.1039/B800154E
Chandra A, Singh B, Upadhyay S, Singh RM (2008) Copper-free Sonogashira coupling of 2-chloroquinolines with phenyl acetylene and quick annulation to benzo[b][1,6]naphthyridine derivatives in aqueous ammonia. Tetrahedron 64:11680–11685. doi:10.1016/j.tet.2008.10.010
Eicher T, Hauptmann S (2003) The chemistry of heterocycles, 2nd edn. Wiley-VCH, Weinheim 316
Michael JP (2007) Quinoline, quinazoline and acridone alkaloids. Nat Prod Rep 24:223–246. doi:10.1039/B509528J
Pearson W, Fang W (2000) Synthesis of benzo-fused 1-azabicyclo[m.n.0]alkanes via the Schmidt reaction: a formal synthesis of gephyrotoxin. J Org Chem 65:7158–7174. doi:10.1021/jo0011383
Wei L, Hsung RP, Sklenicka HM, Gerasyuto AI (2001) A novel and highly stereoselective intramolecular formal [3+3] cycloaddition reaction of vinylogous amides tethered with \(\alpha \),\(\beta \)-unsaturated aldehydes: a formal total synthesis of (+)-gephyrotoxin. Angew Chem Int Ed 40:1516–1518. doi:10.1002/1521-3773(20010417)40:8<1516::AID-ANIE1516>3.0.CO;2-V
Dillard RD, Pavey DE, Benslay DN (1973) Synthesis and antiinflammatory activity of some 2,2-dimethyl-1,2-dihydroquinolines. J Med Chem 16:251–253. doi:10.1021/jm00261a019
Joshi AA, Viswanathan CL (2006) Recent developments in antimalarial drug discovery. Anti Infect Agents Med Chem 5:105–122. doi:10.2174/187152106774755626
Kidwai M, Negi N (1997) Synthesis of some novel substituted quinolines. Monatsh Chem 128:85–89. doi:10.1007/BF00807642
Alqasoumi I, Al-Taweel AM, Alafeefy AM, Noaman E, Ghorab MM (2010) Novel quinolines and pyrimido[4,5-b]quinolines bearing biologically active. Eur J Med Chem 45:738–744. doi:10.1016/j.ejmech.2009.11.021
Baumann M, Baxendale IR (2015) Batch and flow synthesis of pyrrolo[1,2-a]-quinolines. J Org Chem 80:10806–10816. doi:10.1021/acs.joc.5b01982
Sarkar S, Bera K, Jalal S, Jana U (2013) Synthesis of structurally diverse polyfunctional pyrrolo[1,2-\(a\)]quinolines by sequential Iron-catalyzed. Eur J Org Chem 27:6055–6061. doi:10.1002/ejoc.201300659
Glukhareva TV, D’yachenko EV, Morzherin YY (2002) Synthesis of spiro derivatives. Chem Heterocycl Compd 38:1426–1427. doi:10.1023/A:1022107332320
Keivanloo A, Bakherad M, Rahmani M, Rahimi A (2013) A Novel one-pot access to 2-formyl/acetyl-1-substituted pyrrolo[2,3-b]quinoxalines under Sonogashira reaction conditions. Monatsh Chem 144:859–863. doi:10.1007/s00706-012-0887-1
Bakherad M, Keivanloo A, Samangooei S (2012) Synthesis of 1-aryl-substituted-4-chloroimidazo[1,2-a]quinoxalines catalyzed by \(\text{ PdCl }_{2}\) in water. Tetrahedron Lett 23:1447–1449. doi:10.1016/j.tetlet.2012.01.028
Bakherad M, Keivanloo A, Jajarmi S (2012) Synthesis of pyrrolo[2,3-b]quinoxalines by the Pd/C-catalyzed multicomponent reaction of 1,2-dichloroquinoxaline with hydrazine hydrate, phenylacetylene, and a variety of aldehydes in water. Tetrahedron 68:2107–2112. doi:10.1016/j.tet.2012.01.045
Keivanloo A, Bakherad M, Rahimi A, Taheri SAN (2010) One-pot synthesis of 1,2-disubstituted pyrrolo[2,3-b]quinoxalines via palladium-catalyzed heteroannulation in water. Tetrahedron Lett 51:2409–2412. doi:10.1016/j.tetlet.2010.02.123
Meth-Cohn O, Narine B, Tamowski B (1981) A versatile new synthesis of quinolines and related fused pyridines. J Chem Soc Perkin Trans 1:1520–1530. doi:10.1039/P19810001520
Sharma N, Asthana M, Nandini D, Singh RP, Singh RM (2013) An economical nucleophilic route toward facile synthesis of pyrano[4,3-b]quinolin-1-ones via 6-endo-dig cyclization of o-alkynylquinoline esters. Tetrahedron 69:1822–1829. doi:10.1016/j.tet.2012.12.068
Ljungdahl T, Bennur T, Dallas A, Emtenäs H, Mårtensson J (2008) Two competing mechanisms for the copper-free Sonogashira cross-coupling reaction. Organometallics 27:2490–2498. doi:10.1021/om800251s
Acknowledgments
We gratefully acknowledge the financial support of the Research Council of the Shahrood University of Technology.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
11030_2016_9694_MOESM1_ESM.docx
The supporting information for this work is available, as follows: copies of 1H and 13C spectra of all the 1-amino substituted pyrrolo[1,2-a]quinoline-4-carboxylate esters.(doc 1.83MB)
Rights and permissions
About this article
Cite this article
Keivanloo, A., Kazemi, S.S., Nasr-Isfahani, H. et al. Efficient one-pot synthesis of new 1-amino substituted pyrrolo[1,2-a]quinoline-4-carboxylate esters via copper-free Sonogashira coupling reactions. Mol Divers 21, 29–36 (2017). https://doi.org/10.1007/s11030-016-9694-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11030-016-9694-7