Abstract
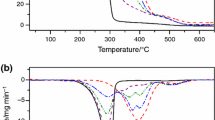
Recent research has revealed the successful use of thermogravimetric analysis (TGA) in dynamic mode as an alternative method for determining degrees of oil conversion into biodiesel. This paper reports the results obtained with the use of TGA in quasi-isothermal mode (TGA-qISO) and with quantitative proton nuclear magnetic resonance (1H qNMR) to investigate the compositions of blends of soybean oil and biodiesels (10:0, 8:2, 6:4, 4:6, 2:8, and 0:10, m:m). Also, reported are data acquired by applying a programmed sequence of TGA-qISO and gas chromatography with flame ionization detection (GC-FID) steps to a biodiesel witness sample and samples prepared by a partner laboratory. All the biodiesels were obtained by transesterification via the methyl route using homogeneous basic catalysis. Thermogravimetric curves (TG) clearly distinguished between mass losses caused by ester volatilization and those resulting from oil thermal decomposition. Ester contents calculated by TGA-qISO proved concordant with 1H qNMR and GC-FID data, with maximum differences of around 0.5% and 1.4%, respectively.




Similar content being viewed by others
References
Gebremariam SN, Marchetti JM. Economics of biodiesel production: review. Energy Convers Manag. 2018. https://doi.org/10.1016/j.enconman.2018.05.002.
Ito K. CO2 emissions, renewable and non-renewable energy consumption, and economic growth: evidence from panel data for developing countries. Int Econ. 2017. https://doi.org/10.1016/j.inteco.2017.02.001.
Teo SH, Islam A, Taufiq-Yap YH. Algae derived biodiesel using nanocatalytic transesterification process. Chem Eng Res Des. 2016. https://doi.org/10.1016/j.cherd.2016.04.012.
Zhang W-B. Review on analysis of biodiesel with infrared spectroscopy. Renew Sustain Energy Rev. 2012. https://doi.org/10.1016/j.rser.2012.07.003.
Ambat I, Srivastava V, Sillanpää M. Recent advancement in biodiesel production methodologies using various feedstock: a review. Renew Sustain Energy Rev. 2018. https://doi.org/10.1016/j.rser.2018.03.069.
Abed KA, Gad MS, El Morsi AK, Sayed MM, Elyazeed SA. Effect of biodiesel fuels on diesel engine emissions. Egypt J Pet. 2019. https://doi.org/10.1016/j.ejpe.2019.03.001.
Knothe G. Analyzing biodiesel: standards and other methods. JAOCS J Am Oil Chem Soc. 2006. https://doi.org/10.1007/s11746-006-5033-y.
Lôbo IP, Ferreira LSC. Biodiesel: parâmetros de qualidade e métodos analíticos. 2009;32:1596–608.
ASSOCIACÃO BRASILEIRA DE NORMAS TÉCNICAS. NBR 15764: Biodiesel—Determinacão do teor total de ésteres por cromatografia gasosa; 2015.
Henderson RK, Jiménez-González C, Constable DJC, Alston SR, Inglis GGA, Fisher G, et al. Expanding GSK’s solvent selection guide: embedding sustainability into solvent selection starting at medicinal chemistry. Green Chem. 2011. https://doi.org/10.1039/c0gc00918k.
Santos AGD, Araujo AS, Caldeira VPS, Fernandes VJ, Souza LD, Barros AK. Model-free kinetics applied to volatilization of Brazilian sunflower oil, and its respective biodiesel. Thermochim Acta. 2010. https://doi.org/10.1016/j.tca.2010.04.015.
Mothé CG, De Castro BCS, Mothé MG. Characterization by TG/DTG/DSC and FTIR of frying and fish oil residues to obtain biodiesel. J Therm Anal Calorim. 2011. https://doi.org/10.1007/s10973-011-1795-z.
Sousa FP, Luciano MA, Pasa VMD. Thermogravimetry and viscometry for assessing the ester content (FAME and FAEE). Fuel Process Technol. 2013. https://doi.org/10.1016/j.fuproc.2012.09.049.
Vega-Lizama T, Díaz-Ballote L, Hernández-Mézquita E, May-Crespo F, Castro-Borges P, Castillo-Atoche A, et al. Thermogravimetric analysis as a rapid and simple method to determine the degradation degree of soy biodiesel. Fuel. 2015. https://doi.org/10.1016/j.fuel.2015.04.047.
Da Silva JCT, Gondim AD, Galvão LPFC, Da Costa Evangelista JP, Araujo AS, Fernandes VJ. Thermal stability evaluation of biodiesel derived from sunflower oil obtained through heterogeneous catalysis (KNO3/Al2O3) by thermogravimetry. J Therm Anal Calorim. 2015. https://doi.org/10.1007/s10973-014-4145-0.
Gaglieri C, Alarcon RT, de Moura A, Mendes RA, Caires FJ. Is thermogravimetry an efficient alternative to gas chromatography in degree of biodiesel conversion? J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7364-y.
Almazrouei M, Elagroudy S, Janajreh I. Transesterification of waste cooking oil: quality assessment via thermogravimetric analysis. Energy Proced. 2019. https://doi.org/10.1016/j.egypro.2019.01.478.
Ionashiro M. Giolito: fundamentos de termogravimetria e análise térmica diferencial/calorimetria exploratória diferencial. 2nd ed. Giz Editorial: São Paulo; 2014.
Verma P, Sharma MP. Review of process parameters for biodiesel production from different feedstocks. Renew Sustain Energy Ver. 2016. https://doi.org/10.1016/j.rser.2016.04.054.
da Silva JCM, Nicolau CL, Cabral MRP, Costa ER, Stropa JM, Silva CAA, et al. Thermal and oxidative stabilities of binary blends of esters from soybean oil and non-edible oils (Aleurites moluccanus, Terminalia catappa, and Scheelea phalerata). Fuel. 2020. https://doi.org/10.1016/j.fuel.2019.116644.
European Committee for Standardization. EN-14103: Determination of ester and linolenic acid methyl ester contents. 2003; 1–11.
Malz F, Jancke H. Validation of quantitative NMR. J Pharm Biomed Anal. 2005. https://doi.org/10.1016/j.jpba.2005.01.043.
De Medeiros EJL, Do Egypto Queiroga RCR, De Souza AG, Cordeiro AMTM, De Medeiros AN, De Souza DL, et al. Thermal and quality evaluation of vegetable oils used in ruminant feed. J Therm Anal Calorim. 2013; doi:https://doi.org/10.1007/s10973-012-2653-3.
Zhang Q, Saleh ASM, Chen J, Sun P, Shen Q. Monitoring of thermal behavior and decomposition products of soybean oil: an application of synchronous thermal analyzer coupled with fourier transform infrared spectrometry and quadrupole mass spectrometry. J Therm Anal Calorim. 2014. https://doi.org/10.1007/s10973-013-3283-0.
Paul AK, Achar SK, Dasari SR, Borugadda VB, Goud VV. Analysis of thermal, oxidative and cold flow properties of methyl and ethyl esters prepared from soybean and mustard oils. J Therm Anal Calorim. 2017. https://doi.org/10.1007/s10973-017-6424-z.
de Oliveira TF, Dweck J. Liquid phase oxidation quantitative analysis of biodiesel/diesel blends by differential TG and DTA. J Therm Anal Calorim. 2018. https://doi.org/10.1007/s10973-018-7298-4.
Miller JN, Miller JC. Statistics and chemometrics for analytical chemistry, vol 46, 6th ed. New York: 2010; doi:https://doi.org/10.1198/tech.2004.s248.
Chand P, Reddy CV, Venkat JG, Wang T, Grewell D. Thermogravimetric quantification of biodiesel produced via alkali catalyzed transesterification of soybean oil. Energy Fuels. 2009. https://doi.org/10.1021/ef800668u.
Acknowledgements
The authors are grateful for the financial support provided by the Mato Grosso do Sul Foundation for the Promotion of Teaching, Science, and Technology Development (Fundect-MS, Brazil) and the Brazilian Council for Scientific and Technological Development (CNPq). This investigation was partly funded by the Coordination for the Improvement of Higher Education Personnel (Capes, Brazil; finance code 001) and by the Universidade Federal de Mato Grosso do Sul.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cortes, M.R., de Queiroz, J.F., dos Santos, T.M.N. et al. Applying quasi-isothermal thermogravimetry to determine degrees of oil conversion into biodiesel. J Therm Anal Calorim 147, 4397–4402 (2022). https://doi.org/10.1007/s10973-021-10834-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10973-021-10834-y