Abstract
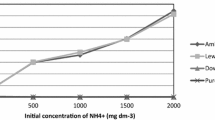
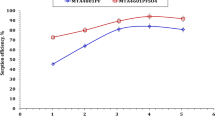
The present work deals with the adsorption of uranium from a nitric acid waste solution using the cation exchange resin Amberjet 1200 H (AHR) . Batch experiments were performed in order to assess the performance of AHR in uranium adsorption. The influences of pH, contact time, initial uranium concentration and temperature have been enhanced. The physical parameters including the adsorption kinetics, the isotherm models and the thermodynamic data have also been determined to determine the nature of the uranium adsorption by AHR. The studied resin has been agreed with both the pseudo second order reaction and Langmuir isotherm.













Similar content being viewed by others
References
Choi J, Lee JY, Yang JS (2009) Biosorption of heavy metals and uranium by starfish and Pseudomonas putida. Hazard Mater 15:157–162
Metilda P, Sanghamitra K, Gladis JM, Naidu GRK, Rao TP (2005) Amberlite XAD-4 functionalized with succinic acid for the solid phase extractive preconcentration and separation of uranium(VI). Talanta 65:192–200
Donat R, Cılgı GK, Aytas S, Cetisl H (2009) Thermodynamics parameters and sorption of U(VI) on ACSD. Radioanal Nucl Chem 279:271–280
Merritt RC (1971) The extractive metallurgy of uranium. Colorado School of Mines Research Institute, Chicago
Streat M, Naden D (1987) Ion exchange and sorption processes in hydrometallurgy. Critical reports on applied chemistry, vol 19. Wiley, New York
Guettaf H, Becis A, Ferhat K, Hanou K, Bouchiha D, Yakoubi K, Ferrad F (2009) Concentration-purification of uranium from an acid leaching solution. Phys. Proc. 2:765–771
Khawassek YM (2014) Production of commercial uranium concentrate from El-Sela Shear zone mineralized ore material, South Eastern Desert- Egypt, at Inshas Pilot plant unit. Nuclear Sciences Scientific Journal 3:169–179
Abdel Aal M (2014) Purification of uranium concentrate from Abo- Rushied ore material with emphasize upon ion exchange technique, South Eastern Desert, Egypt, Faculty of science Ain Shams University
Mirjial K, Roshani M (2007) Resin-in-pulp method for uranium recovery from leached pulp of low grade uranium ore. Hydrometallurgy 85(2–4):103–107
Kraus KA, Moor GE (1950) Adsorption of protactinium from hydrochloric acid solutions by anion exchange Resins. J Am Chem Soc 72(9):4293–4294
Abd El-Ghany MS, Mahdy MA, Abd ElMonem NM, El-Hazek NT (1994) Pilot plant studies on the treatment of E1 Atshan uranium ores, Eastern Desert, Egypt. 2nd Arab conference peaceful uses of atomic energy, AAEA, Cairo
Barnes CD, da Silva Neves R A, Streat M (1974) Anion exchange of uranium from aqueous sulphuric acid solutions: diffusion kinetics. J Appl Chem Biotechnol 24:787–801
Abd El-Ghany MS (2000) Uranium recovery from sulfate leach pulps of some Egyptian Ores. Ph.D., Faculty of Engineering, Cairo University
Cheira MF, El-Didamony AM, Mahmoud KF, Atia BM (2014) Equilibrium and kinetic characteristics of uranium recovery by the strong base Ambersep 920U Cl Resin. IOSR J Appl Chem 7(5):32–40
Bai Y, Bartkiewicz B (2009) Removal of cadmium from wastewater using ion exchange resin Amberjet 1200H columns. Pol J Environ Stud 18(6):1191–1195
Rengaraj S, Kim Y, Joo CK, Choi K, Yi J (2004) Batch adsorptive removal of copper ions in aqueous solutions by ion exchange resins: 1200H and IRN97H. Korean J Chem Eng 21(1):187–194
Zewail TM, Yousef NS (2015) Kinetic study of heavy metal ions removal by ion exchange in batch conical air spouted bed. Alexandria Eng J 54(1):83–90
Marczenko Z, Balcerzak M (2000) Separation, preconcentration and spectrophotometry in inorganic analysis. Elsevier Science B.V., Amsterdam, p 521
Rohwer H, Rheeder N, Hosten E (1997) Interactions of uranium and thorium with arsenazoIII in an aqueous medium. Anal Chim Acta 341(2):263–268
Davies W, Gray W (1964) A rapid and specific volumetric method for the precise determination of uranium using ferrous sulfate as a reductant”. Talanta 11:1203–1211
Mathew KJ, Mason B, Morales ME, Narayann UI (2009) Uranium assay determination using Davies and Gray titration: an overview and implementation of GUM uncertainty evaluation. Radio Anal Nucl Chem 282:939–944
Puigdomenech I (2006) HYDRA (hydrochemical equilibrium-constant database) and MEDUSA (make equilibrium diagrams using sophisticated algorithms) programs, Royal Institute of Technology, Sweden. http://www.kemi.kth.se/medusa/
Chen Y, Wu F, Lin YX, Deng NS, Bazhin N, Glebov E (2007) Photodegradation of glyphosate in the ferrioxalate system. J Hazard Mater 148:360–365
Sheng L, Zhou L, Huang Z, Liu Z, Chen Q, Huang G, Adesina AA (2016) Facile synthesis of magnetic chitosan nano-particles functionalized with N/O-containing groups for efficient adsorption of U(VI) from aqueous solution. J Radioanal Nucl Chem 310:1361–1371
Chegrouche S, Mellah A, Telmoune S (1997) Removal of lanthanum from aqueous solutions by natural bentonite. Water Res 31:1733–1737
Mellah A, Chegrouche S (1997) The removal of zinc from aqueous solutions by natural bentonite. Water Res 31:621–629
Bhatnagar A, Jain AK (2005) A comparative adsorption study with different industrial wastes as adsorbents for the removal of cationic dyes from water. J Colloid Interface Sci 28(1):49–55
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Li L, Ding DX, Hu N, Fu PK, Xin X, Wang YD (2014) Adsorption of U(VI) ions from low concentration uranium solution by thermally activated sodium feldspar. J Radioanal Nucl Chem 299:681–690
Manes M, Hofer BJE (1969) Application of the Polanyi adsorption potential theory to adsorption from solution on activated carbon. J Phys Chem 73(3):584–590
Abderrahim O, Didi MA, Villemin D (2009) A new sorbent for uranium extraction polyethyleniminephenylphosphonamidic acid. J Radioanal Nucl Chem 279(1):237–244
Ansari SA, Mohapatra PK, Manchanda VK (2009) A novel malonamide grafted polystyrene- divinyl benzene resin for extraction pre-concentration and separation of actinides. J Hazard Mater 161(2–3):1323–1329
Metilda P, Sanghamitra K, Gladis JM, Naidu GRK, Rao TP (2005) Amberlite XAD-4 functionalized with succinic acid for the solid phase extractive preconcentration and separation of uranium(VI). Talanta 65(1):192–200
Venkatesan K, Sukumaran V, Antony M, Vasudeva Rao P (2004) Extraction of uranium by amine, amide and benzamide grafted covalently on silica gel. J Radioanal Nucl Chem 260(3):443–450
Camacho LM, Deng S, Parra RR (2010) Uranium removal from groundwater by natural clinoptilolite zeolite: effects of pH and initial feed concentration. J Hazard Mater 175(1–3):393–398
Ferrah N, Abderrahim O, Didi MA (2015) Comparative study of Cd2+ ions sorption by both Lewatit TP214 and Lewatit TP 208 resins: kinetic. Equilib Thermodyn Model Chem J 5(1):6–13
Lagergren S (1898) Zur theorie der sogenannten adsorption gelo ¨ster stoffe Kungliga venska Vetenskapsakademiens. Handlingar 24(4):1–39
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Srinivasan T, Rao P, Sood D (1997) The effect of temperature on the extraction of uranium(VI) from nitric acid by tri-n-amyl phosphate. Solvent Extr Ion Exch 15:15–31
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Khawassek, Y.M., Masoud, A.M., Taha, M.H. et al. Kinetics and thermodynamics of uranium ion adsorption from waste solution using Amberjet 1200 H as cation exchanger. J Radioanal Nucl Chem 315, 493–502 (2018). https://doi.org/10.1007/s10967-017-5692-1
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10967-017-5692-1