Abstract
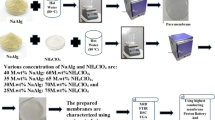
The investigation on bio-based polymer electrolytes (BBPEs) system based on alginate doped with a various composition of glycolic acid (GA) were carried out and prepared using solution casting technique. The BBPEs complexes were characterized by using fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), thermal gravimetric analysis (TGA), differential scanning calorimetry (DSC) and electrical impedance spectroscopy (EIS). The complexation was observed to have taken place between alginate and GA with apparent changes of the peak wavenumber, specifically at the –COO− of alginate functional group. Moreover, from the impedance analysis, it is evident that the sample which contains 20 wt. % of GA possessed the optimum ionic conductivity of 5.32 × 10−5 S cm−1 at room temperature with the lowest activation energy. The ionic conductivity increased by incorporating GA was demonstrated via the enhancement of their thermal stability as well as amorphousness. The findings of the present investigation suggest that alginate polymer has the potential to be applied as an electrolyte system for electrochemical devices applications.













Similar content being viewed by others
References
Milczarek G, Inganäs O (2012) Renewable cathode materials from biopolymer/conjugated polymer interpenetrating networks. Science 335(6075):1468–1471. https://doi.org/10.1126/science.1215159
Singh R, Bhattacharya B, Tomar SK, Singh V, Singh PK (2017) Electrical, optical and electrophotochemical studies on agarose based biopolymer electrolyte towards dye sensitized solar cell application. Measurement 102:214–219. https://doi.org/10.1016/j.measurement.2017.02.014
Pawlicka A, Firmino A, Vieira D, Sentanin F, Grote JG, Kajzar F Gelatin-and DNA-based ionic conducting membranes for electrochromic devices. In: Optical Materials in Defence Systems Technology VI, 2009. International Society for Optics and Photonics, p 74870J. https://doi.org/10.1117/12.835913
Teoh KH, Lim C-S, Liew C-W, Ramesh S (2015) Electric double-layer capacitors with corn starch-based biopolymer electrolytes incorporating silica as filler. Ionics 21(7):2061–2068. https://doi.org/10.1007/s11581-014-1359-x
Karthikeyan S, Selvasekarapandian S, Premalatha M, Monisha S, Boopathi G, Aristatil G, Arun A, Madeswaran S (2017) Proton-conducting I-carrageenan-based biopolymer electrolyte for fuel cell application. Ionics 23(10):2775–2780. https://doi.org/10.1007/s11581-016-1901-0
Croisier F, Jérôme C (2013) Chitosan-based biomaterials for tissue engineering. Eur Polym J 49(4):780–792. https://doi.org/10.1016/j.eurpolymj.2012.12.009
Tan YM, Lim SH, Tay BY, Lee MW, Thian ES (2015) Functional chitosan-based grapefruit seed extract composite films for applications in food packaging technology. Mater Res Bull 69:142–146. https://doi.org/10.1016/j.materresbull.2014.11.041
Ahmad NH, Isa MIN Structural and ionic conductivity studies of CMC based polymerelectrolyte doped with NH4Cl. In: Advanced Materials Research, 2015. Trans Tech Publ, pp 247–252. https://doi.org/10.4028/www.scientific.net/AMR.1107.247
Du JF, Bai Y, Pan DA, Chu WY, Qiao LJ (2009) Characteristics of proton conducting polymer electrolyte based on chitosan acetate complexed with CH3COONH4. J Polym Sci B Polym Phys 47(6):549–554. https://doi.org/10.1002/polb.21656
Khiar ASA, Arof AK (2010) Conductivity studies of starch-based polymer electrolytes. Ionics 16(2):123–129. https://doi.org/10.1007/s11581-009-0356-y
Ahmad AH Electrical analysis of cornstarch-based polymer electrolyte doped with NaCl. In: Solid state phenomena, 2017. Trans Tech Publ, pp 347–351. https://doi.org/10.4028/www.scientific.net/SSP.268.347
Mobarak NN, Ramli N, Ahmad A, Rahman MYA (2012) Chemical interaction and conductivity of carboxymethyl κ-carrageenan based green polymer electrolyte. Solid State Ionics 224:51–57. https://doi.org/10.1016/j.ssi.2012.07.010
Yang J-M, Wang N-C, Chiu H-C (2014) Preparation and characterization of poly (vinyl alcohol)/sodium alginate blended membrane for alkaline solid polymer electrolytes membrane. J Membr Sci 457:139–148. https://doi.org/10.1016/j.memsci.2014.01.034
Lacoste C, El Hage R, Bergeret A, Corn S, Lacroix P (2018) Sodium alginate adhesives as binders in wood fibers/textile waste fibers biocomposites for building insulation. Carbohydr Polym 184:1–8. https://doi.org/10.1016/j.carbpol.2017.12.019
Lu Y, Wang R, He J, Xu P, Li H, Tian L, Guo C (2019) Synthesis of bimetallic CoMn-alginate and synergistic effect on thermal decomposition of ammonium perchlorate. Mater Res Bull 117:1–8. https://doi.org/10.1016/j.materresbull.2019.04.013
Treenate P, Monvisade P, Yamaguchi M (2014) Development of hydroxyethylacryl chitosan/alginate hydrogel films for biomedical application. J Polym Res 21(12):601. https://doi.org/10.1007/s10965-014-0601-6
Yu C, Li X, Liu Z, Yang X, Huang Y, Lin J, Zhang J, Tang C (2016) Synthesis of hierarchically porous TiO2 nanomaterials using alginate as soft templates. Mater Res Bull 83:609–614. https://doi.org/10.1016/j.materresbull.2016.07.014
Salama HE, Aziz MSA, Sabaa MW (2018) Novel biodegradable and antibacterial edible films based on alginate and chitosan biguanidine hydrochloride. Int J Biol Macromol 116:443–450. https://doi.org/10.1016/j.ijbiomac.2018.04.183
Aziz SB, Woo TJ, Kadir MFZ, Ahmed HM (2018) A conceptual review on polymer electrolytes and ion transport models. Journal of Science: Advanced Materials and Devices 3(1):1–17. https://doi.org/10.1016/j.jsamd.2018.01.002
Ngai KS, Ramesh S, Ramesh K, Juan JC (2016) A review of polymer electrolytes: fundamental, approaches and applications. Ionics 22(8):1259–1279. https://doi.org/10.1007/s11581-016-1756-4
Shukur MF, Kadir MFZ (2015) Electrical and transport properties of NH4Br-doped cornstarch-based solid biopolymer electrolyte. Ionics 21(1):111–124. https://doi.org/10.1007/s11581-014-1157-5
Salleh NS, Aziz SB, Aspanut Z, Kadir MFZ (2016) Electrical impedance and conduction mechanism analysis of biopolymer electrolytes based on methyl cellulose doped with ammonium iodide. Ionics 22(11):2157–2167. https://doi.org/10.1007/s11581-016-1731-0
Monisha S, Mathavan T, Selvasekarapandian S, Benial AMF (2017) Preparation and characterization of cellulose acetate and lithium nitrate for advanced electrochemical devices. Ionics 23(10):2697–2706. https://doi.org/10.1007/s11581-016-1886-8
Kumar LS, Selvin PC, Selvasekarapandian S, Manjuladevi R, Monisha S, Perumal P (2018) Tamarind seed polysaccharide biopolymer membrane for lithium-ion conducting battery. Ionics:1–11. https://doi.org/10.1007/s11581-018-2541-3
Sudhakar YN, Selvakumar M, Bhat DK (2015) Preparation and characterization of phosphoric acid-doped hydroxyethyl cellulose electrolyte for use in supercapacitor. Materials for Renewable and Sustainable Energy 4(3):10. https://doi.org/10.1007/s40243-015-0051-z
Fadzallah IA, Majid SR, Careem MA, Arof AK (2014) A study on ionic interactions in chitosan–oxalic acid polymer electrolyte membranes. J Membr Sci 463:65–72. https://doi.org/10.1016/j.memsci.2014.03.044
Senthilkumar P, Ganesh T, Vinoth K, Maria Sylvester M, Anand Karunakaran DJS, Hudge P, Kumbharakhane AC (2019) Dielectric relaxation and molecular interaction investigation of glycolic acid-water mixture using time domain reflectometry. Indian Journal of Pure & Applied Physics (IJPAP) 57(3):180–187
Bakhshi A, Bhalla G (2004) Electrically conducting polymers: materials of the twentyfirst century
Samsudin AS, Isa MIN (2012) Structural and electrical properties of carboxy methylcellulose-dodecyltrimethyl ammonium bromide-based biopolymer electrolytes system. Int J Polymer Mater 61(1):30–40. https://doi.org/10.1080/00914037.2011.557810
Dey A, Karan S, Dey A, De SK (2011) Structure, morphology and ionic conductivity of solid polymer electrolyte. Mater Res Bull 46(11):2009–2015. https://doi.org/10.1016/j.materresbull.2011.07.008
Ranjana PAB, Jeya S, Abarna S, Premalatha M, Arulsankar A, Sundaresan B (2019) Enhancement of Na+ ion conduction in polymer blend electrolyte P (VdF-HFP)–PMMA-NaTf by the inclusion of EC. J Polym Res 26(2):38. https://doi.org/10.1007/s10965-019-1704-x
Zainuddin NK, Samsudin AS (2018) Investigation on the effect of NH4Br at transport properties in K–carrageenan based biopolymer electrolytes via structural and electrical analysis. Mater Today Comm 14:199–209. https://doi.org/10.1016/j.mtcomm.2018.01.004
Rasali NMJ, Samsudin AS (2017) Ionic transport properties of protonic conducting solid biopolymer electrolytes based on enhanced carboxymethyl cellulose-NH4Br with glycerol. Ionics:1–12. https://doi.org/10.1007/s11581-017-2318-0
Aprilliza M. Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. In: IOP Conference Series: Materials Science and Engineering, 2017. vol 1. IOP Publishing, p 012019. https://doi.org/10.1088/1757-899X/188/1/012019
Hu Y, Zhang S, Han D, Ding Z, Zeng S, Xiao X (2018) Construction and evaluation of the hydroxypropyl methyl cellulose-sodium alginate composite hydrogel system for sustained drug release. J Polym Res 25(7):148. https://doi.org/10.1007/s10965-018-1546-y
Fawzy MA, Gomaa M, Hifney AF, Abdel-Gawad KM (2017) Optimization of alginate alkaline extraction technology from Sargassum latifolium and its potential antioxidant and emulsifying properties. Carbohydr Polym 157:1903–1912. https://doi.org/10.1016/j.carbpol.2016.11.077
Bardajee GR, Hooshyar Z, Soleyman R (2017) Nanocomposites of sodium alginate biopolymer and CdTe/ZnS quantum dots for fluorescent determination of amantadine. J Polym Res 24(8):128. https://doi.org/10.1007/s10965-017-1247-y
Botvin V, Latypov A, Ponarin N, Filimoshkin A. Synthesis of glycolide by catalytic depolymerization of glycolic acid oligomers modified by polyhydric alcohols. In: Journal of Physics: Conference Series, 2019. vol 1. IOP Publishing, p 012019. https://doi.org/10.1088/1742-6596/1145/1/012019,
Varghese HT, Panicker CY (2011) IR, Raman and Ab-initio Calcualtions of glycolic acid. Orient J Chem 27(1):215–220
Ramlli MA, Bashirah NAA, Isa MIN Ionic Conductivity and Structural Analysis of 2-hyroxyethyl Cellulose Doped with Glycolic Acid Solid Biopolymer Electrolytes for Solid Proton Battery. In: IOP Conference Series: Materials Science and Engineering (2018) vol 1. IOP Publishing, p 012038. https://doi.org/10.1088/1757-899X/440/1/012038
Hay MB, Myneni SCB (2007) Structural environments of carboxyl groups in natural organic molecules from terrestrial systems. Part 1: infrared spectroscopy. Geochim Cosmochim Acta 71(14):3518–3532. https://doi.org/10.1016/j.gca.2007.03.038
Ahokas JM, Kosendiak I, Krupa J, Wierzejewska M, Lundell J (2019) Raman spectroscopy of glycolic acid complexes with N2. J Mol Struct 1183:367–372. https://doi.org/10.1016/j.molstruc.2019.01.080
Sikkanthar S, Karthikeyan S, Selvasekarapandian S, Pandi DV, Nithya S, Sanjeeviraja C (2015) Electrical conductivity characterization of polyacrylonitrile-ammonium bromide polymer electrolyte system. J Solid State Electrochem 19(4):987–999. https://doi.org/10.1007/s10008-014-2697-3
Lopes S, Bueno L, AGUIAR JÚNIOR FD, Finkler C (2017) Preparation and characterization of alginate and gelatin microcapsules containing lactobacillus rhamnosus. Anais da academia Brasileira de Ciências (AHEAD):0-0. https://doi.org/10.1590/0001-3765201720170071
Ramlli MA, Isa MIN (2016) Structural and ionic transport properties of protonic conducting solid biopolymer electrolytes based on Carboxymethyl cellulose doped with ammonium fluoride. J Phys Chem B 120(44):11567–11573. https://doi.org/10.1021/acs.jpcb.6b06068
Huang X, Zheng Q, Fang X, Shao H (2018) Probing qualitative change in intermolecular forces from hydrogen bonding to electrostatic interaction on the surface of self-assembled monolayer. J Electrochem Soc 165(5):H240–H246. https://doi.org/10.1149/2.0731805jes
Bakhtin S, Shved E, Bespal'ko Y (2017) Nucleophile-electrophile interactions in the reaction of oxiranes with carboxylic acids in the presence of tertiary amines. J Phys Org Chem 30(12). https://doi.org/10.1002/poc.3717
Monisha S, Mathavan T, Selvasekarapandian S, Benial AMF, Aristatil G, Mani N, Premalatha M (2017) Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. Carbohydr Polym 157:38–47. https://doi.org/10.1016/j.carbpol.2016.09.026
Samsudin AS, Khairul WM, Isa MIN (2012) Characterization on the potential of carboxy methylcellulose for application as proton conducting biopolymer electrolytes. J Non-Cryst Solids 358(8):1104–1112. https://doi.org/10.1016/j.jnoncrysol.2012.02.004
Samsudin AS, Lai HM, Isa MIN (2014) Biopolymer materials based carboxymethyl cellulose as a proton conducting biopolymer electrolyte for application in rechargeable proton battery. Electrochim Acta 129:1–13. https://doi.org/10.1016/j.electacta.2014.02.074
Chai MN, Isa MIN (2013) The oleic acid composition effect on the carboxymethyl cellulose based biopolymer electrolyte. Journal of Crystallization Process and Technology 3(01):1–4. https://doi.org/10.4236/jcpt.2013.31001
Alakanandana A, Subrahmanyam AR, Kumar JS (2016) Structural and electrical conductivity studies of pure PVA and PVA doped with succinic acid polymer electrolyte system. Mater, Today: Proceedings 3(10):3680–3688. https://doi.org/10.1016/j.matpr.2016.11.013
Rikukawa M, Sanui K (2000) Proton-conducting polymer electrolyte membranes based on hydrocarbon polymers. Prog Polym Sci 25(10):1463–1502. https://doi.org/10.1016/S0079-6700(00)00032-0
Liew C-W, Ramesh S (2013) Studies on ionic liquid-based corn starch biopolymer electrolytes coupling with high ionic transport number. Cellul 20(6):3227–3237. https://doi.org/10.1007/s10570-013-0079-0
Qu X, Wirsen A, Albertsson A-C (2000) Effect of lactic/glycolic acid side chains on the thermal degradation kinetics of chitosan derivatives. Polym 41(13):4841–4847. https://doi.org/10.1016/S0032-3861(99)00704-1
Rani MSA, Rudhziah S, Ahmad A, Mohamed NS (2014) Biopolymer electrolyte based on derivatives of cellulose from kenaf bast fiber. Polym 6(9):2371–2385. https://doi.org/10.3390/polym6092371
Saadiah MA, Zhang D, Nagao Y, Muzakir SK, Samsudin AS (2019) Reducing crystallinity on thin film based CMC/PVA hybrid polymer for application as a host in polymer electrolytes. J Non-Cryst Solids 511:201–211. https://doi.org/10.1016/j.jnoncrysol.2018.11.032
Shukur MF, Ithnin R, Kadir MFZ (2014) Electrical properties of proton conducting solid biopolymer electrolytes based on starch–chitosan blend. Ionics 20(7):977–999. https://doi.org/10.1007/s11581-013-1033-8
Mazuki NF, Fuzlin AF, Saadiah MA, Samsudin AS (2018) An investigation on the abnormal trend of the conductivity properties of CMC/PVA-doped NH4Cl-based solid biopolymer electrolyte system. Ionics:1–11. https://doi.org/10.1007/s11581-018-2734-9
Samsudin AS, Saadiah MA (2018) Ionic conduction study of enhanced amorphous solid bio-polymer electrolytes based carboxymethyl cellulose doped NH4Br. J Non-Cryst Solids 497:19–29. https://doi.org/10.1016/j.jnoncrysol.2018.05.027
Chitra R, Sathya P, Selvasekarapandian S, Monisha S, Moniha V, Meyvel S (2018) Synthesis and characterization of iota-carrageenan solid biopolymer electrolytes for electrochemical applications. Ionics 25:1–11. https://doi.org/10.1007/s11581-018-2687-z
Li X, Xie H, Lin J, Xie W, Ma X (2009) Characterization and biodegradation of chitosan–alginate polyelectrolyte complexes. Polym Degrad Stab 94(1):1–6. https://doi.org/10.1016/j.polymdegradstab.2008.10.017
Perumal P, Selvin PC, Selvasekarapandian S (2018) Characterization of biopolymer pectin with lithium chloride and its applications to electrochemical devices. Ionics 24(10):3259–3270. https://doi.org/10.1007/s11581-018-2507-5
Ghosal K, Das A, Das SK, Mahmood S, Ramadan MAM, Thomas S (2019) Synthesis and characterization of interpenetrating polymeric networks based bio-composite alginate film: a well-designed drug delivery platform. Int J Biol Macromol 130:645–654. https://doi.org/10.1016/j.ijbiomac.2019.02.117
Rezvanian M, Ahmad N, Amin MCIM, Ng S-F (2017) Optimization, characterization, and in vitro assessment of alginate-pectin ionic cross-linked hydrogel film for wound dressing applications. Int J Biol Macromol 97:131–140. https://doi.org/10.1016/j.ijbiomac.2016.12.079
Sampathkumar L, Selvin PC, Selvasekarapandian S, Perumal P, Chitra R, Muthukrishnan M (2019) Synthesis and characterization of biopolymer electrolyte based on tamarind seed polysaccharide, lithium perchlorate and ethylene carbonate for electrochemical applications. Ionics 25(3):1067–1082. https://doi.org/10.1007/s11581-019-02857-1
Rasali NMJ, Nagao Y, Samsudin AS (2018) Enhancement on amorphous phase in solid biopolymer electrolyte based alginate doped NH4NO3. Ionics:1–14. https://doi.org/10.1007/s11581-018-2667-3
Kadir MFZ, Hamsan MH (2018) Green electrolytes based on dextran-chitosan blend and the effect of NH4SCN as proton provider on the electrical response studies. Ionics 24(8):2379–2398. https://doi.org/10.1007/s11581-017-2380-7
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Boopathi G (2018) Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J Non-Cryst Solids 481:424–434. https://doi.org/10.1016/j.jnoncrysol.2017.11.027
Gao C, Pollet E, Avérous L (2017) Properties of glycerol-plasticized alginate films obtained by thermo-mechanical mixing. Food Hydrocoll 63:414–420. https://doi.org/10.1016/j.foodhyd.2016.09.023
Larosa C, Salerno M, de Lima JS, Meri RM, da Silva MF, de Carvalho LB, Converti A (2018) Characterisation of bare and tannase-loaded calcium alginate beads by microscopic, thermogravimetric, FTIR and XRD analyses. Int J Biol Macromol 115:900–906. https://doi.org/10.1016/j.ijbiomac.2018.04.138
Choe SR, Haldorai Y, Jang S-C, Rethinasabapathy M, Lee Y-C, Han Y-K, Jun Y-S, Roh C, Huh YS (2018) Fabrication of alginate/humic acid/Fe-aminoclay hydrogel composed of a grafted-network for the efficient removal of strontium ions from aqueous solution. Environ Technol Innov 9:285–293. https://doi.org/10.1016/j.eti.2017.12.008
Yusof YM, Illias HA, Kadir MFZ (2014) Incorporation of NH4Br in PVA-chitosan blend-based polymer electrolyte and its effect on the conductivity and other electrical properties. Ionics 20(9):1235–1245. https://doi.org/10.1007/s11581-014-1096-1
Samsudin AS, Isa MIN (2012) Structural and ionic transport study on CMC doped NH4Br: a new types of biopolymer electrolytes. J Appl Sci 12(2):174–179. https://doi.org/10.3923/jas.2012.174.179
Kadir MFZ, Salleh NS, Hamsan MH, Aspanut Z, Majid NA, Shukur MF (2018) Biopolymeric electrolyte based on glycerolized methyl cellulose with NH4Br as proton source and potential application in EDLC. Ionics 24(6):1651–1662. https://doi.org/10.1007/s11581-017-2330-4
Karthikeyan S, Sikkanthar S, Selvasekarapandian S, Arunkumar D, Nithya H, Kawamura J (2016) Structural, electrical and electrochemical properties of polyacrylonitrile-ammonium hexaflurophosphate polymer electrolyte system. J Polym Res 23(3):51. https://doi.org/10.1007/s10965-016-0952-2
Shukur MF, Ibrahim FM, Majid NA, Ithnin R, Kadir MFZ (2013) Electrical analysis of amorphous corn starch-based polymer electrolyte membranes doped with LiI. Phys Scr 88(2):025601. https://doi.org/10.1088/0031-8949/88/02/025601
Kadir MFZ, Majid SR, Arof AK (2010) Plasticized chitosan–PVA blend polymer electrolyte based proton battery. Electrochim Acta 55(4):1475–1482. https://doi.org/10.1016/j.electacta.2009.05.011
Aziz NAN, Idris NK, Isa MIN (2010) Proton conducting polymer electrolytes of methylcellulose doped ammonium fluoride: conductivity and ionic transport studies. Int J Phy Sci 5(6):748–752. https://doi.org/10.5897/IJPS
Fuzlin AF, Rasali NMJ, Samsudin AS. Effect on ammonium bromide in dielectric behavior based alginate solid biopolymer electrolytes. In: IOP Conference Series: Materials Science and Engineering, 2018. vol 1. IOP Publishing, p 012080. https://doi.org/10.1088/1757-899X/342/1/012080
Tripathy T, Kolya H, Jana S (2018) Selective lead (II) adsorption and flocculation characteristics of the grafted sodium alginate: a comparative study. J Polym Environ 26(3):926–937. https://doi.org/10.1007/s10924-017-1004-7
Othman L, Isa KBM, Osman Z, Yahya R (2017) Ionic transport studies of gel polymer electrolytes containing sodium salt. Mater, Today: Proceedings 4(4):5122–5129. https://doi.org/10.1016/j.matpr.2017.05.017
Premalatha M, Mathavan T, Selvasekarapandian S, Selvalakshmi S, Monisha S (2017) Incorporation of NH4Br in tamarind seed polysaccharide biopolymer and its potential use in electrochemical energy storage devices. Org Electron 50:418–425. https://doi.org/10.1016/j.orgel.2017.08.017
Mohan VM, Qiu W, Shen J, Chen W (2010) Electrical properties of poly (vinyl alcohol)(PVA) based on LiFePO 4 complex polymer electrolyte films. J Polym Res 17(1):143–150. https://doi.org/10.1007/s10965-009-9300-0
Kim D-S, Woo JC, Youk JH, Manuel J, Ahn J-H (2014) Gel polymer electrolytes based on nanofibrous polyacrylonitrile–acrylate for lithium batteries. Mater Res Bull 58:208–212. https://doi.org/10.1016/j.materresbull.2014.01.047
Sinha R, Basu S, Meikap AK (2018) The investigation of the electrical transport properties of Gd doped YCrO3 nanoparticles. Mater Res Bull 97:578–587. https://doi.org/10.1016/j.materresbull.2017.08.055
Sohaimy MIH, Isa MIN (2017) Ionic conductivity and conduction mechanism studies on cellulose based solid polymer electrolytes doped with ammonium carbonate. Polym Bull 74(4):1371–1386. https://doi.org/10.1007/s00289-016-1781-5
Kopitzke RW, Linkous CA, Anderson HR, Nelson GL (2000) Conductivity and water uptake of aromatic-based proton exchange membrane electrolytes. J Electrochem Soc 147(5):1677–1681. https://doi.org/10.1149/1.1393417
Fuzlin AF, Nagao Y, Misnon II, Samsudin AS (2019) Studies on structural and ionic transport in biopolymer electrolytes based on alginate-LiBr. Ionics:1–16. https://doi.org/10.1007/s11581-019-03386-7
Chai MN, Isa MIN (2016) Novel proton conducting solid bio-polymer electrolytes based on carboxymethyl cellulose doped with oleic acid and plasticized with glycerol. Sci Rep 6:27328. https://doi.org/10.1038/srep27328
Sohaimy MIH, Isa MIN (2015) Effect of ammonium carbonate salt concentration on structural and ionic conductivity of cellulose based solid polymer electrolytes. Fibers Polym 16(5):1031–1034. https://doi.org/10.1007/s12221-015-1031-8
Acknowledgements
The authors would like to thank Ministry of High Education (MOHE) for FRGS (FRGS/1/2019/STG07/UMP/02/4: RDU 1901114), Faculty of Industrial Science & Technology for the research support and Universiti Malaysia Pahang for providing Master Research Scheme (MRS).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Fuzlin, A., Saadiah, M., Yao, Y. et al. Enhancing proton conductivity of sodium alginate doped with glycolic acid in bio-based polymer electrolytes system. J Polym Res 27, 207 (2020). https://doi.org/10.1007/s10965-020-02142-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10965-020-02142-0