Abstract
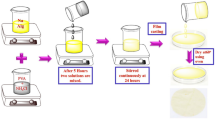
Biopolymer electrolyte based on sodium alginate (NaAlg) with various concentrations of NH4ClO4 has been prepared by solution casting technique using double distilled water as solvent. Influence of different concentrations of NH4ClO4 on the biopolymer NaAlg is systematically investigated by the different characterization techniques such as XRD, FTIR, DSC, TGA, electrical impedance spectroscopy analysis, transference number measurement, and LSV. XRD was used to investigate whether the prepared biopolymers were crystalline or amorphous. The formation of complexes between NaAlg and NH4ClO4 has been observed by FTIR. Using differential scanning calorimeter, glass transition temperature (Tg) is found for the prepared biopolymer electrolyte. The maximum ionic conductivity of 3.59 × 10−3 S cm−1 has been obtained for 30 M.wt% NaAlg:70 M.wt% NH4ClO4 biopolymer electrolyte using AC impedance analysis. Using DC Wagner’s polarization technique, the ionic transference number has been determined. The highest conducting biopolymer electrolyte's electrochemical stability window was found by the LSV technique to be 2.71 V. The primary proton battery and proton exchange membrane (PEM) fuel cell have been constructed using highest ionic conducting biopolymer membrane (30 M.wt% NaAlg:70 M.wt% NH4ClO4), and the performance has been studied. The proton battery’s open circuit voltage (Voc) is 1.76 V, while the PEM fuel cell’s open circuit voltage (Voc) is 789 mV.
Graphical Abstract





















Similar content being viewed by others
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to the manuscript is not yet published, but are available from the corresponding author on reasonable request.
References
Kim JG, Son B, Mukherjee S, Schuppert N, Bates A, Kwon O, Choi MJ, Chung HY, Park S (2015) A review of lithium and non lithium based solid state batteries. J Power Sources 282:299–322
Khan NM, Ali NSM, Fuzlin AF, Samsudin AS (2020) Ionic conductiviy of alginate-NH4Cl polymer electrolyte. Makara J Technol 24(3):5
Liu YH, Zhu LQ, Shi Yi, Wan Q (2014) Proton conducting sodium alginate electrolyte laterally coupled low- voltage oxide – based transistors. Appl Phys Lett 104(133504):1–4
Gao H, Lian K (2014) Proton –conducting polymer electrolytes and their applications in solid supercapacitors a review. J RSC Adv 4:33091–33113
Fuzlin AF, Saadiah MA, Yao Y, Nagao Y, Samsudin AS (2020) Enhancing proton conductivity of sodium alginate doped with glycolic acid in bio-based polymer electrolytes system. J Polym Res 207:1–16
Premalatha M, Mathavan T, Selvasekarapandian S, Monisha S, Pandi DV, Selvalakshmi S (2016) Investigations on proton conducting biopolymer membranes based on tamarind seed polysaccharide incorporated with ammonium thiocyanate. J Non-Cryst Solids 453:131–140
Draget KI, Skjak-Braek G, Christensen BE, Gaserod O, Smidsrod O (1996) Swelling and partial solubilization of alginic acid gel beads in acidic buffer. CarbohydratePolym 29:209–215
Yeom CK, Lee KH (1998) Characterization of sodium alginate membrane crosslinked with glutaraldehyde in pervaporation separation. J Appl Polym Sci 67:209–219
Salisu A, MohdMarsinSanagi AA, Naim WA, Ibrahim W, Karim KA (2015) Removal of lead ions from aqueous solutions using sodium alginate-graft-poly (methyl methacrylate) beads. J Desalination and Water Treatment 57:15353–15361
Oliveira Filho JG, Rodrigues JM, Valadares ACF, Almeida AB, Lima TM, Takeuchi KP (2019) Active food packaging: alginate films with cottonseed protein hydrolysates. Food Hydrocolloids 92:267–275
Akin Alper, NuranIsiklan, (2016) Microwave assisted synthesis and characterization of sodium alginate-graft-poly (N, N-dimethylacrylamide). Int J of Biol Macromol 82:530–540
Chen W, Feng Q, Zhang G, Yang Q, Zhang C (2017) The effect of sodium alginate on the flotation separation of scheelite from calcite and fluorite. Miner Eng 113:1–7
Bae SB, Nam HC, Park WH (2019) Electro spraying of environmentally sustainable alginate microbeads for cosmetic additives. Int J Biol Macromol 133:278–283
Sreekanth Reddy O, Subha MCS, Jithendra T, Madhavi C, Chowdoji Rao K (2020) Curcumin encapsulated dual cross linked sodium alginate/montmorillonite polymeric composite beads for controlled drug delivery. J Pharmaceutical Anal 20:31022–31024
Satheeshbabu BK, Mohamed I (2015) Synthesis and characterization of sodium alginate conjugate and study of effect of conjugation on drug release from matrix tablet. Indian J Pharm Sci 77:579–585
Reddy PRS, Rao KM, Rao KSVK (2014) Synthesis of alginate based silver nanocomposite hydrogels for biomedical applications. Macromol Res 22:832–842
Li J, He J, Huang Y (2017) Role of alginate in antibacterial finishing of textiles. Int J of Biol Macromol 94:466–473
Iwaki YO, Hernandezescalona M, Briones JR, Pawlicka A (2012) Sodium alginate based ionic conducting membranes. Mol Cryst Liq Cryst 554:221–231
Mohanapriya S, Bhat SD, Sahu AK, Manokaran A, Vijayakumar R, Pitchumani S, Sridhar P, Shukla AK (2010) Sodium alginate based proton exchange membranes as electrolyte for DMFCs. Energy Environ Sci 3:1746–1756
Jansi R, Shenbagavalli S, Revathy MS, Deepalakshmi S, Indumathi P, Mohammed KA (2023) Structural and ionic transport in biopolymer electrolyte-based PVA:NaAlg with NH4Cl for electrochemical applications. J.Materials science: Materials in Electronics 34:963
Diana MI, Lakshmi D, Christopher Selvin P, Selvasekarapandian S (2022) Substantial ion conduction in the biopolymer membrane: efficacy of NaI on sodium alginate matrix. J Materials letters 312:131652
Diana MI, Selvasekarapandian S, Christopher Selvin P, Vengadesh Krishna M (2022) A physicochemical elucidation of sodium perchlorate incorporated alginate biopolymer: toward all-solid-state sodium-ion battery. J Materials Science 57:8211–8224
Diana MI, Christopher Selvin P, Selvasekarapandian S, Vengadesh Krishna M (2021) Investigations on Na-ion conducting electrolyte based on sodium alginate biopolymer for all-solid-state sodium-ion batteries. J Solid State Electrochem 25:2009–2020
Tamilisai R, Palanisamy PN, Selvasekarapandian S, Maheshwari T (2021) Sodium alginate incorporated with magnesium nitrate as a novel solid biopolymer electrolyte for magnesiumion batteries. J Mater Sci: Mater Electron 32:22270–22285
Christopher Selvin P, Perumal P, Selvasekarapandian S, Monisha S, Boopathi G, Leena Chandra MV (2018) Study of proton-conducting polymer electrolyte based on K-carrageenan and NH4SCN for electrochemical devices. Ionics 24:3535–3542
Vanitha N, Shanmugapriya C, Selvasekarapandian S, Naachiyar RM, Krishna MV, Aafrin S, Nandhini K (2022) Effect of graphene quantum dot on sodium alginate with ammonium formate (NH4HCO2) biopolymer electrolytes for the application of electrochemical devices. Ionics 28:2731–2749
Selvalakshmi S, Mathavan T, Selvasekarapandian S, Premalatha M (2018) A study of electrochemical devices based on Agar-Agar-NH4I biopolymer electrolytes. AIP conference proceedings 1942(1):140019
Selvalakshmi S, Mathavan T, Selvasekarapandian S, Premalatha M (2018) Effect of ethylene carbonate plasticizer on agar-agar: NH4Br-based solid polymer electrolytes. Ionics 24:2209–2217
Aziz SB, Brza MA, Saed SR, Hamsan MH, Kadir MFZ (2020) Ion association as a main shortcoming in polymer blend electrolytes based on GS:PS incorporated with various amounts of ammonium tetrafluoroborate. J Mater Res Technol 9:5410–5421
Aziz SB, Nofal MM, Rebar T, Abdulwahid KMFZ, Hadi JM, Hessien MM, Kareem WO, Dannoun EMA, SaeedImpedance SR (2021) FTIR and transport properties of plasticized proton conducting biopolymer electrolyte based on chitosan for electrochemical device application. J Results in Physics 29:104770
Sohaimy MIH, Natural IMIN (2020) Inspired carboxymethyl cellulose (CMC) doped with ammonium carbonate (AC) as biopolymer electrolyte. J Polymers 12:2487
Sohaimy MIH, Isa MIN (2022) Proton-conducting biopolymer electrolytes based on carboxymethyl cellulose doped with ammonium formate. J Polymers 14:3019
Ramlli MA, Isa MINM, Kamarudin KH (2022) 2-Hydroxyethyl cellulose-ammonium thiocyanate solid biopolymer electrolytes: ionic conductivity and dielectric studies. J Sustain Sci Management 17:121–132
Muthukrishnan M, Shanthi C, Selvasekarapandian S, Shanthi G, Sampathkumar L, Maheshwari T (2021) Impact of ammonium formate (AF) and ethylene carbonate (EC) on the structural, electrical, transport and electrochemical properties of pectin-based biopolymer membranes. Ionics 27:3443–3459
Muthukrishnan M, Shanthi C, Selvasekarapandian S, Premkumar R (2023) Biodegradable flexible proton conducting solid biopolymer membranes based on pectin and ammonium salt for electrochemical applications. Int J Hydrogen Energy 48:5387–5401
Maheshwari T, Tamilarasan K, Selvasekarapandian S, Chitra R, Kiruthika S (2021) Investigation of blend biopolymer electrolytes based on dextran-PVA with ammonium thiocyanate. J Solid State Electrochem 25:755–765
Meera Naachiyar R, Ragam M, Selvasekarapandian S, Aristatil AafrinHazaana, Muniraj Vignesh N, Vengadesh Krishna M (2022) Fabrication of rechargeable proton battery and PEM fuel cell using biopolymer gellan gum incorporated with NH4HCO2 solid electrolyte. J Polym Res 29:337
Vanitha N, Shanmugapriya C, Selvasekarapandian S, VengadeshKrishna NK (2022) Investigation of N-S-based graphene quantum dot on sodium alginate with ammonium thiocyanate (NH4SCN) biopolymer electrolyte for the application of electrochemical devices. J Materials Sci: Materials Electronics 33:14847–14867
Hajifathaliha F, Mahboubi A, Nematollahi L, Mohit E, Bolourchian N (2018) Comparison of different cationic polymers efficacy in fabrication of alginate multilayer microcapsules. Asian J Pharmaceutical Sciences 15:95–103
Rasali NMJ, Samsudin AS (2018) Characterization on ionic conductivity of solid bio-polymer electrolytes system based alginate doped ammonium nitrate via impedance spectroscopy. AIP conference proceeding 2020:1–8
Hodge RM, Edward GH, Simon GP (1996) Water absorption and states of water in semicrystalline poly(vinyl alcohol) films. Polymer 37:1371–1376
Sridevi D, Rajendran KV (2009) Synthesis and optical characteristics of ZnO nanocrystals. Bull Master Sci 32(2):165–168
Vij A, Chawla AK, Kumar R, Lochab SP, Chandra R, Singh N (2010) Effect of 120 MeV Ag9+ ion beam irradiation on the structure and photoluminescence of SrS: Ce nanostructures. Phys B 405(11):2573–2576
Mangalam R, Thamilselvan M, Selvasekarapandian S, Jayakumar S, Manjuladevi R (2017) Magnesium ion conducting polyvinyl alcohol–polyvinyl pyrrolidone-based blend polymer electrolyte. Ionics 23:1771–1781
Zhi J, Tian-Fang W, Shu-Fen L, Feng-Qi Z, Zi-Ru L, Cui-Mei Y, Yang L, Shang-Wen L, Gang-Zhui Z (2006) Thermal behavior of ammonium perchlorate and metal powders of different grades. J Therm Anal Calorim 85:315–320
Helmiyati AM (2017) Characterization and properties of Sodium alginate from brown algae used as an ecofriendly superabsorbent. IOP Conf Ser Mater Sci Eng 188:12019
Fuzlin AF, Bakri NA, Sahraoui B, Samsudin AS (2020) Study on the effect of lithium nitrate in ionic conduction properties based alginate biopolymer electrolytes. Mater Res Express 7:015902
Aprilliza M (2017) Characterization and properties of sodium alginate from brown algae used as an ecofriendly superabsorbent. IOP Conf Ser Mater Sci Eng 188:12019
Kanti P, Srigowri K, Madhuri J, Smitha B, Sridhar S (2004) Dehydration of ethanol through blend membranes of chitosan and sodium alginate by pervaporation. Sep Puri Technol 40:259–266
Moniha V, Marimuthu A, Selvasekarapandian S, Sundaresan B, Hemalatha R (2019) Development and characterization of biopolymer electrolyte iota-carrageenan with ammonium salt for electrochemical application. Mater Today Proc 8:449–455
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Hemalatha R, Boopathi G (2018) Synthesis and characterization of bio-polymer electrolyte based on iota-carrageenan with ammonium thiocyanate and its applications. J Solid State Electrochem 22:3209–3223
Maheshwari T, Tamilarasan K, Selvasekarapandian S, Chitra R, Muthukrishnan M (2021) Synthesis and characterization of dextran, poly (vinyl alcohol) blend biopolymer electrolytes with NH4NO3, for electrochemical applications. Int J Green Energy 19:314–330
Fuzlin AF, Samsudin AS (2021) Studies on favorable ionic conduction and structural properties of biopolymer electrolytes system-based alginate. J Polym Bull 78:2155–2175
Boukamp BA (1986) A nonlinear least square fit procedure for analysis of impedance data of electrochemical systems. Solid State Ionics 20:31–44
Karthikeyan S, Sikkanthar S, Selvasekarapandian S, Arunkumar D, Nithya H, Iwa Y, Kawamura J (2016) Structural, electrical and electrochemical properties of polyacrylonitrile-ammonium hexaflurophosphate polymer electrolyte system. J Polym Res 23:51
Rasali NMJ, Nagao Y, Samsudin AS (2019) Enhancement on amorphous phase in solid biopolymer electrolyte based alginate doped NH4NO3. Journal of Ionics 25:641–654
Moniha V, Alagar M, Selvasekarapandian S, Sundaresan B, Boopathi G (2018) Conductive bio-polymer electrolyte iota-carrageenan with ammonium nitrate for application in electrochemical devices. J Non-Cryst Solids 481:424–434
Monisha S, Mathavan T, Selvasekarapandian S, Milton Franklin Benial A, Aristatil G, Mani N, Premalatha M, Vinoth Pandi D (2017) Investigation of bio polymer electrolyte based on cellulose acetate-ammonium nitrate for potential use in electrochemical devices. CarbohydrPolym 157:38–47
Hashmi SA, Chandra S (1995) Experimental investigations on a sodium- ion- conducting polymer electrolyte based on poly(ethylene oxide) complexed with NaPF6. Mater Sci Eng B 34:18–26
Wagner JB, Wagner CJ (1957) Electrical conductivity measurements on curprous halides. J Chem Phys 26:1597–1601
Mahalakshmi M, Selvanayagam S, Selasekarapandian S, Monisha V (2019) Characterization of biopolymer electrolytes based on cellulose acetate with magnesium perchlorate (Mg(ClO4)2) for energy storage devices. J Sci Adv Materials Devices 4:276–284
Selvalakshmi S, Mathavan T, Selvasekarapandian S, Premalatha M (2019) Characterization of biodegradable solid polymer electrolyte system based on agar-NH4Br and its comparison with NH4I. J Solid State Electrochem 23:1727–1737
Pandey K, Lakshmi N, Chandra S (1998) A rechargeable solid state proton battery with an intercalating cathode and an anode containing a hydrogen storage-material. J Power Sources 76(1):116–123
Meera Naachiyar R, Ragam M, Selvasekarapandian S, Vengadesh Krishna M, Buvaneshwari P (2021) Development of biopolymer electrolyte membrane using gellan gum biopolymer incorporated with NH4SCN for electro-chemical application. J Ionics 27:3415–3429
Tang Y, Yuan W, Pan M, Wan Z (2010) Feasibility study of porous copper fiber sintered felt: a novel porous flow field in proton exchange membrane fuel cells. Int J Hydrogen Energy 35:9661–9677
Author information
Authors and Affiliations
Contributions
Entire work has been done by N. Vanitha, and full manuscript has been written by N. Vanitha. The full manuscript has been corrected by C. Shanmugapriya. The concept of the work is given by S. Selvasekarapandian. FTIR study has been done by Muniraj Vignesh N. Linear sweep voltammetry study has been done by Aafrin Hazaana S. DSC analysis has been done by Meera Naachiyar R. Fuel cell construction work has been done by Kamatchi Devi S.
Corresponding author
Ethics declarations
Ethics approval
Not applicable.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
N, V., C, S., S, S. et al. Synthesis of biopolymer electrolyte using sodium alginate with ammonium perchlorate (NH4ClO4) for the application of electrochemical devices. Ionics 29, 4037–4054 (2023). https://doi.org/10.1007/s11581-023-05115-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11581-023-05115-7