Abstract
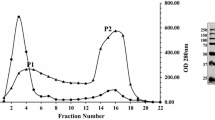
A β-1,3-endoglucanase produced by Streptomyces rutgersensis was purified to a homogeneity by the fractional precipitation with ammonium sulfate, ion exchange chromatography on Q-Sepharose and hydrophobic chromatography on Butyl Sepharose. A typical procedure provided 11.74-fold purification with 12.53 % yield. SDS-PAGE of the purified protein showed one protein band. The exact molecular mass of the enzyme obtained by mass spectrometry was 41.25 kDa; the isoelectric point was between pH 4.2–4.4. The optimal β-glucanase catalytic activity was at pH 7 and 50 °C. An enzyme was only active toward glucose polymers containing β-1,3 linkages and hydrolyzed Saccharomyces cerevisiae cell wall β-glucan in an endo-like way: reaction products were different molecular size β-glucans, which were larger than glucose.







Similar content being viewed by others
Abbreviations
- AU:
-
Unit of enzyme activity
- ESI:
-
Electrospray ionization
- Km :
-
Michaelis–Menten constant
- MS:
-
Mass spectrometry
- pI:
-
Isoelectric point
- QTOF:
-
Quadrupole time-of-flight mass spectrometer
- RPM:
-
Revolutions per minute
- SDS-PAGE:
-
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- SEC:
-
Size-exclusion chromatography
- TLC:
-
Thin layer chromatography
- Vmax :
-
Maximum velocity
References
Aguilar-Uscanga B, François JM (2003) Lett Appl Microbiol 37:268–274
Bacic A, Fincher GB, Stone BA (2009) Chemistry, biochemistry, and biology of 1–3 beta glucans and related polysaccharides. Academic Press, Burlington
Balasubramanian V, Vashisht D, Cletus J, Sakthive N (2012) Biotechnol Lett 34(11):1983–1990
Beyer M, Diekmann H (1984) Appl Microbiol Biotechnol 20(3):207–212
Chan GC, Chan WC, Man-Yuen SDD (2009) J Hematol Oncol 2:25
Chen J, Seviour R (2007) Mycol Res 3:635–652
De Marco JL, Felix CR (2007) Braz Arch Biol Technol 50:21–29
Doxey AC, Yaish MWF, Moffatt BA, Griffith M, McConkey BJ (2007) Mol Biol Evol 24(4):1045–1055
DuBois M, Gilles KA, Hamilton JK, Rebers PA, Smith F (1956) Anal Chem 28:350–356
Hong TY, Cheng CW, Huang JW, Meng M (2002) Microbiology 148:1151–1159
Huber CN, Scobell H, Tai H (1966) Cereal Chem 43:342–346
Hunter JKW, Gault RA, Berner MD (2002) Lett Appl Microbiol 35:267–271
Javmen A, Grigiškis S, Gliebutė R (2012) Biologija 58(2):51–59
Krah M, Misselwitz R, Politz O, Thomsen KK, Welfle H, Borriss R (1998) Eur J Biochem 257:101–111
Li H, Chen J, Li A, Li DC (2007) World J Microbiol Biotechnol 23:1297–1303
Lowry OH, Rosebrough N, Farr AL, Randall RJ (1951) J Biol Chem 193(1):265–275
Martin K, McDougall BM, McIlroy S, Jayus JC, Seviour RJ (2007) FEMS Microbiol Rev 31:168–192
Miller GL (1959) Anal Chem 31:426–428
Noroha FE, Ulhoa CJ (2000) FEMS Microbiol Lett 183:119–123
Novak M, Vetvicka V (2008) J Immunotoxicol 5:47–57
Pang Z, Otaka K, Suzuki Y, Goto K, Ohnishi M (2004) J Biol Macromol 4:57–66
Park JK, Kim JD, Park YI, Kim SK (2012) Carbohydr Polym 87(2):1641–1648
Pelizon AC, Kaneno R, Soares AMVC, Meira DA, Sartori A (2005) Physiol Res 54:557–564
Petravić-Tominac V, Zechner-Krpan V, Grba S, Panjkota-Krbavčić I, Vidović L (2010) Agric Conspec Sci 75:149–158
Rop O, Mlcek J, Jurikova T (2009) Nutr Rev 67:624–631
Shokri H, Asadi F, Khosravi AR (2008) Nat Prod Res 22:414–421
Sun L, Gurnon JR, Adams BJ, Graves MV, Van Etten LJ (2000) Virology 276:27–36
Tanabe YY, Oda MM (2011) Prot Proteomics 1814(12):7
Vetvicka V (2011) World J Clin Oncol 2:115–119
Vieira FA, da Cunha M, Klein DE, Carvalho AO, Gomes VM (2006) Braz Arch Biol Technol 49(6):881–888
Yi SY, Hwang BK (1997) Mol Cells 143:1701–1708
Zhou L, Zhu YT, Chen GJ, Liu WF (2011) Microbiol China 38:839–846
Zverlov VV, Volkov I, Velikodvorskaya TV, Schwarz W (1997) Microbiology 143:1701–1708
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Javmen, A., Grigiškis, S., Rudenkov, M. et al. Purification and Partial Characterization of a Novel β-1,3-Endoglucanase from Streptomyces rutgersensis . Protein J 32, 411–417 (2013). https://doi.org/10.1007/s10930-013-9500-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10930-013-9500-7