Abstract
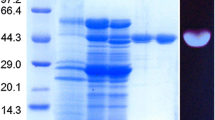
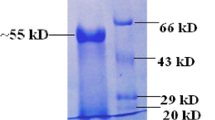
A thermostable extracellular β-1,3-glucanase from Chaetomium thermophilum was purified to homogeneity by fractional ammonium sulfate precipitation, Pheny1-Sepharose hydrophobic interaction chromatography, ion exchange chromatography on DEAE-Sepharose and gel filtration on Sephacryl S-100. SDS-PAGE of the purified enzyme showed a single protein band of molecular weight 76.3 kDa. The enzyme exhibited optimum catalytic activity at pH 6.0 and 60 °C. It was thermostable at 50 °C, and retained 90% activity after 60 min at 60 °C. The half-life at 65 °C, 70 °C and 80 °C was 55 min, 21.5 min, and 5 min, respectively. The N-terminal amino acid sequence (8 residues) of the enzyme was HWLGDIPH. The HPLC analysis showed that the only enzymatic product formed from laminarin by the purified β-1,3-glucanase was glucose, indicating that the enzyme is an exo-β-1,3-glucanase (EC 3.2.1.58).





Similar content being viewed by others
References
Ait-Lahsen H, Soler A, Rey M, De La Cruz J, Monte E, Llobell A (2001) An antifungal exo-α-1,3-glucanase (AGN13.1) from the biocontrol fungus Trichoderma harzianum. Appl Environ Microbiol 67:5833–5839
Anna AK, Karl KT, Konstantin AS, Irina AS, Elena VE, Andrew NS, Kirill NN (2001) Isolation, enzymatic properties, and mode of action of an exo-1,3-β-glucanase from T. viride. Eur J Biochem 268:6123–6131
Aono R, Hammuram M, Yamamoto M, Asano T (1995) Isolation of extracellular 28- and 42 kilodalton β-1,3-glucanases and comparison of three β-1,3-glucanases produced by Bacillus circulans IAM1165. Appl Environ Microbiol 61:122–129
Archambault C, Coloccia P, Kermasha S, Jabaji-Hare S (1998) Characterization of an endo-1,3-(-D-glucanase produced during the interaction between the mycoparasite Stachybotrys elegans and its host Rhizoctonia solani. Can J Microbiol 44:989–997
Benny C, Yona C, Yitzhak H (1998) Purification and characterization of laccase from Chaetomium thermophilum and its role in humification. Appl Environ Microbiol 64:3175–3179
Bergmeyer HU, Bernt E (1974) Determination of glucose with oxidase and peroxidase. Method Enzym Anal 3:1205–1215
Chen J, Li DC, Zhang YQ, Zhou QX (2005) Purification and characterization of a thermostable glucoamylase from Chaetomium thermophilum. J General Appl Microbiol 51:175–181
Cohen-Kupiec R, Broglie KE, Friesem D, Broglie RM, Chet I (1999) Molecular characterization of a novel β-1,3-exoglucanase related to mycoparasitism of Trichoderma harzianum. Gene 226:147–154
De La Cruz J, Pintor-Toro JA, Benítez T, Llobell A, Romero LC (1995) A novel endo-β-1,3-glucanase, BGN13.1, involved in the mycoparasitism of Trichoderma harzianum. J Bacteriol 177:6937–6945
Fontaine T, Hartland R, Beauvais A, Diaquin M, Latgé JP (1997) Purification and characterization of an endo-1,3-β-glucanase from Aspergillus fumigatus. Eur J Biochem 243:315–321
Ganju RK, Vithayathil PJ, Murthy SK (1989) Purification and characterization of two xylanase from Chaetomium thermophile var. coprophile. Can J Microbiol 35:836–842
Gueguen Y, Voorhorst WGB, Van Der Oost J, De Vos WM (1997) Molecular and biochemical characterization of an endo-β-1,3-glucanase of the hyperthermophilic archaeon Pyrococcus furiosus. J Biol Chem 272:31258–31264
Hien NH, Fleet GH (1983) Separation and characterization of six (1-3)-β-glucanases from Saccharomyces cerevisiae. J Bacteriol 156:1204–1213
Hoj PB, Hartman DJ, Morrice NA, Doan DN, Fincher GB (1989) Purification of (1, 3)-β-glucan endohydrolase isoenzyme II from germinated barley and determination of its primary structure from a cDNA clone. Plant Mol Biol 13:31–42.
Hrmova M, Fincher GB (1993) Purification and properties of three (1-3)-β-D-glucanase isoenzymes from young leaves of barley (Hordeum vulgare). Biochem J 289:453–461
Jirku V, Kraxnerova B, Krumphanzl V (1980) The extracellular system of (-1,3-glucanases of Alternaria tenuissima and Aspergillus versicolor. Folia Microbiol 25:24–31
Konig J, Grasser R, Pikor H, Vogel K (2002) Determination of xylanase, β-glucanase, and cellulase activity. Anal Bioanal Chem 374:80–87
Krah M, Misselwitz R, Politz O, Thomsen KK, Welfle H, Borriss R (1998) The laminarinase from thermophilic eubacterium Rhodothermus marinus – conformation, stability, and identification of active site carboxylic residues by site-directed mutagenesis. Eur J Biochem 257:101–111
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of the bacteriophage T4. Nature 227:680–685
Li DC, Lu M, Li YL, Lu J (2003) Purification and characterization of an endocellulase from the thermophilic fungus Chaetomium thermophilum CT2. Enzyme Microb Technol 33:932–938
Mannonen L, Ritala A, Nuutila A M, Kurten U, Aspegren K, Teeri TH, Aikasalo R, Tammisola J, Kauppinen V (1997) Thermotolerant fungal glucanase in malting barley. Proceedings 26th Congress European Brewery Convention, Maastricht, pp 91–100
Mohagheghpour N, Dawson M, Hobbs P, Judd A, Winant R, Dousman L, Waldeck N, Hokama L, Tuse D, Kos F (1995) Glucans as immunological adjuvants. Adv Exp Med Biol 383:13–22
Pang Z, Otaka K, Maoka T, Hidaka K, Ishijima S, Oda M, Ohnishi M (2005) Structure of β-glucan oligomer from laminarin and its effect on human monocytes to inhibit the proliferation of U937 cells. Biosci Biotechnol Biochem 69:553–858
Pitson SM, Seviour RJ, Mcdougall BM (1993) Noncellulytic fungal β-glucanase: their physiology and regulation. Enzyme Microb Technol 15:178–192
Reichelt BY, Fleet GH (1981) Isolation, properties, function and regulation of endo-(1-3)-β-glucanases in Schizosaccharomyces pombe. J Bacteriol 147:1085–1094
Rios-Hernandez M, Dos-Santos N, Silvia-Cardoso J, Bello-Garciga JL, Pedroso M (1994) Immunopharmacological studies of β-1,3-glucan. Arch Med Res 25:179–180
Rotem Y, Yarden O, Sztejnberg A (1999) The mycoparasite Ampelomyces quisqualis expresses exgA encoding an exo-β-1,3-glucanase in culture and during mycoparasitism. Phytopathology 89:631–638
Ryan EM, Ward OP (1985) Study of the effect of β-1,3-glucanase from basidiomycete QM 806 on yeast extract production. Biotechnol Lett 7:409–412
Schaeffer HJ, Leykam J, Walton JD (1994) Cloning and targeted gene disruption of EXG1, encoding exo-β-1,3-glucanase, in the phytopathogenic fungus Cochliobolus carbonum. Appl Environ Microbiol 60:594–598
Stone BA, Clarke AE (1992) Chemistry and biology of (1–3)-β-glucans. Bundoora, La Trobe University Press, Australia
Sun L, Gurnon JR, Adams BJ, Graves MV, Van Etten JL (2000) Characterization of a β-1,3-glucanase encoded by chlorella virus PBCV-1. Virology 276:27–36
Tangarone B, Royer JC, Nakas JP (1989) Purification and characterization of an endo-(1,3)-β-D-glucanase from Trichoderma longibrachiatum. Appl Environ Microbiol 55:177–184
Tweddell RJ, Jabaji-Hare SH, Goetghebeur M, Charest PM, Kermasha S (1995) Purification and partial characterization of a (-1,3-glucanase secreted by the mycoparasite Stachybotrys elegans. Biosci Biotechnol Biochem 59:2223–2227
Van De Rhee MD, Mendes O, Werten MW, Huizing HJ, Mooibroek H (1996) Highly efficient homologous integration via tandem exo-β-1,3-glucanase genes in the common mushroom, Agaricus bisporus. Curr Genet 30:166–173
Vazquez-Garciduenas S, Leal-Morales CA, Herrera-Estrella A (1998) Analysis of the β-1,3-glucanolytic system of the biocontrol agent Trichoderma harzianum. Appl Environ Microbiol 64:1442–1446
Acknowledgments
This work was supported by the Chinese National Nature Science Foundation and Chinese National Programs for High Technology Research and Development (863).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, H., Chen, J., Li, A. et al. Purification and partial characterization of β-1,3-glucanase from Chaetomium thermophilum . World J Microbiol Biotechnol 23, 1297–1303 (2007). https://doi.org/10.1007/s11274-007-9366-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11274-007-9366-y