Abstract
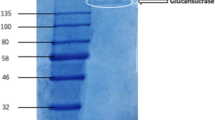
Endo-β-1,3-glucanase (Endo23) was purified from a Trichoderma reesei GIMCC 3.498 fermentation broth using anion exchange and 2-stage size exclusion chromatography. Purification of 44.5× and a 12% recovery yield of enzyme activity were achieved. The Mw and isoelectric point were estimated to be 24 kDa and 3.85 using SDS-PAGE and IEF, respectively. The highest substrate specificity was observed for water-insoluble curdlan. The optimal conditions for hydrolyzing curdlan were pH 5.0 and 50°C. The main hydrolytic products were glucobiose and glucotriose. Minor amounts of glucose and glucotetraose were detected. Hg2+, Fe2+, Fe3+, and Sn2+ inhibited the hydrolysis activity of Endo23 at 5 and 50 mM. K+ slightly promoted Endo23 activity. Endo23 belongs to the category EC3.2.1.39. The peptide sequences of Endo23 showed identity with conserved sequences that typically exist in β-1,3-glucanases of the glycoside hydrolase family. The Endo23 sequence was partially similar to a hypothetical lignocellulase from Penicillium oxalicum 114-2.
Similar content being viewed by others
Refrerences
Bacic A, Fincher GB, Stone BA. Chemistry, Biochemistry, and Biology of (1→3)-β-Glucans and Related Polysaccharides. Elsevier, Amsterdam, the Netherlands. pp. 75–90 (2009)
da Silva Aires R, Steindorff AS, Ramada MHS, de Siqueira SJL, Ulhoa CJ. Biochemical characterization of a 27 kDa 1,3-β-D-glucanase from Trichoderma asperellum induced by cell wall of Rhizoctonia solani. Carbohyd. Polym. 8(7): 1219–1223 (2012)
Duan F, Lu X. Enzymatic properties and kinetics of an endo-β-1,3- glucanase of Mitsuaria chitosanitabida H12 and preparation of 1,3-β-D-glucooligosaccharides from yeast β-glucan. Ann. Microbiol. 6(2): 307–312 (2012)
Pang Z, Otaka K, Maoka T, Hidaka K, Ishijima S, Oda M, Ohnishi M. Structure of β-glucan oligomer from laminarin and its effect on human monocytes to inhibit the proliferation of U937 cells. Biosci. Biotech. Bioch. 6(9): 553–558 (2005)
Klarzynski O, Plesse B, Joubert J-M, Yvin J-C, Kopp M, Kloareg B, Fritig B. Linear β-1,3-glucans are elicitors of defense responses in tobacco. Plant Physiol. 12(4): 1027–1038 (2000)
Borjihan G, Zhong G, Baigude H, Nakashima H, Uryu T. Synthesis and anti-HIV activity of 6-amino-6-deoxy-(1→3)-β-D-curdlan sulfate. Polym. Advan. Technol. 1(4): 326–329 (2003)
Tanabe Y, Oda M. Molecular characterization of endo-1,3-β-glucanase from Cellulosimicrobium cellulans: Effects of carbohydrate-binding module on enzymatic function and stability. BBA-Proteins Proteom. 181(4): 1713–1719 (2011)
Ueda M, Yamaki K, Goto T, Nakazawa M, Miyatake K, Sakaguchi M, Inouye K. Purification and characterization of 1,3-β-D-glucanase from Eisenia foetida. Carbohyd. Polym. 8(6): 271–276 (2011)
Cantarel BL, Coutinho PM, Rancurel C, Bernard T, Lombard V, Henrissat B. The Carbohydrate-active EnZymes database (CAZy): An expert resource for glycogenomics. Nucleic Acids Res. 3(7): 233–238 (2009)
Kikuchi T, Shibuya H, Jones JT. Molecular and biochemical characterization of an endo-β-1,3-glucanase from the pinewood nematode Bursaphelenchus xylophilus acquired by horizontal gene transfer from bacteria. Biochem. J. 38(9): 117–125 (2005)
Hua C, Yi H, Jiao L. Cloning and expression of the endo-1,3(4)-β-glucanase gene from Paecilomyces sp. FLH30 and characterization of the recombinant enzyme. Biosci. Biotech. Bioch. 7(5): 1807–1812 (2011)
Fontaine T, Hartland RP, Beauvais A, Diaquin M, Latge J-P. Purification and characterization of an endo-1,3-β-glucanase from Aspergillus fumigatus. Eur. J. Biochem. 24(3): 315–321 (1997)
Zakharenko AM, Kusaykin MI, Kovalchuk SN, Anastyuk SD, Ly BM, Sova VV, Rasskazov VA, Zvyagintseva TN. Enzymatic and molecular characterization of an endo-1,3-ß-D-glucanase from the crystalline styles of the mussel Perna viridis. Carbohyd. Res. 34(6): 243–252 (2011)
Akiyama T, Pillai MA, Sentoku N. Cloning, characterization and expression of OsGLN2, a rice endo-1,3-β-glucanase gene regulated developmentally in flowers and hormonally in germinating seeds. Planta 22(0): 129–139 (2004)
Nishimura T, Bignon C, Allouch J, Czjzek M, Darbon H, Watanabe T, Henrissat B. Streptomyces matensis laminaripentaose hydrolase is an ‘inverting’ β-1,3-glucanase. FEBS Lett. 49(9): 187–190 (2001)
Genta FA, Terra WR, Ferreira C. Action pattern, specificity, lytic activities, and physiological role of five digestive β-glucanases isolated from Periplaneta americana. Insect Biochem. Mol. Biol. 3(3): 1085–1097 (2003)
Hong T-Y, Meng M. Biochemical characterization and antifungal activity of an endo-1,3-β-glucanase of Paenibacillus sp. isolated from garden soil. Appl. Microbiol. Biotechnol. 6(1): 472–478 (2003)
Shi P, Yao G, Yang P, Li N, Luo H, Bai Y, Wang Y, Yao B. Cloning, characterization, and antifungal activity of an endo-1,3-β-Dglucanase from Streptomyces sp. S27. Appl. Microbiol. Biotechnol. 8(5): 1483–1490 (2010)
Zhan X-B, Lin C-C, Zhang H-T. Recent advances in curdlan biosynthesis, biotechnological production, and applications. Appl. Microbiol. Biotechnol. 9(3): 525–531 (2012)
Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 22(7): 680–685 (1970)
Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 7(2): 248–254 (1976)
Sakamoto Y, Nakade K, Konno N. Endo-β-1,3-glucanase GLU1, from the fruiting body of Lentinula edodes, belongs to a new glycoside hydrolase family. Appl. Environ. Microbiol. 77: 8350–8354 (2011)
Cota J, Alvarez TM, Citadini AP, Santos CR, de Oliveira Neto M, Oliveira RR, Pastore GM, Ruller R, Prade RA, Murakami MT. Mode of operation and low-resolution structure of a multi-domain and hyperthermophilic endo-β-1,3-glucanase from Thermotoga petrophila. Biochem. Biophys. Res. Commun. 40(6): 590–594 (2011)
Hong T-Y, Cheng C-W, Huang J-W, Meng M. Isolation and biochemical characterization of an endo-1,3-β-glucanase from Streptomyces sioyaensis containing a C-terminal family 6 carbohydratebinding module that binds to 1,3-β-glucan. Microbiology+ 14(8): 1151–1159 (2002)
Shu C-H, Xu C-J, Lin E-S. Production, purification, and partial characterization of a novel endo-β-1,3-glucanase from Agaricus brasiliensis. Process Biochem. 4(1): 1229–1233 (2006)
Kestwal RM, Bagal-Kestwal D, Chiang BH. 1,3-β-Glucanase from Vigna aconitifolia and its possible use in enzyme bioreactor fabrication. Int. J. Biol. Macromol. 4(9): 894–899 (2011)
El-Katatny M, Gudelj M, Robra K-H, Elnaghy M, Gübitz G. Characterization of a chitinase and an endo-β-1,3-glucanase from Trichoderma harzianum Rifai T24 involved in control of the phytopathogen Sclerotium rolfsii. Appl. Microbiol. Biotechnol. 5(6): 137–143 (2001)
Akiyama T, Pillai MA. Molecular cloning, characterization, and in vitro expression of a novel endo-1,3-β-glucanase up-regulated by ABA and drought stress in rice (Oryza sativa L.). Plant Sci. 16(1): 1089–1098 (2001)
Lépagnol-Descamps V, Richard C, Lahaye M, Potin P, Yvin J-C, Kloareg B. Purification and determination of the action pattern of Haliotis tuberculata laminarinase. Carbohyd. Res. 31(0): 283–289 (1998)
Akiyama T, Shibuya N, Hrmova M, Fincher GB. Purification and characterization of a (1→3)-β-D-glucan endohydrolase from rice (Oryza sativa) bran. Carbohyd. Res. 29(7): 365–374 (1997)
Nobe R, Sakakibara Y, Fukuda N, Yoshida N, Ogawa K, Suiko M. Purification and characterization of laminaran hydrolases from Trichoderma viride. Biosci. Biotech. Bioch. 6(7): 1349–1357 (2003)
Pesentseva MS, Kusaykin MI, Anastyuk SD, Sova VV, Zvyagintseva TN. Catalytic properties and mode of action of endo- (1→3)-β-D-glucanase and β-D-glucosidase from the marine mollusk Littorina kurila. Carbohyd. Res. 34(3): 2393–2400 (2008)
Martín-Cuadrado A-B, Fontaine T, Esteban P-F, Dedo JEd, Medina- Redondo Md, Rey Fd, Latgé JP, Aldana CR. Characterization of the endo-β-1,3-glucanase activity of S. cerevisiae Eng2 and other members of the GH81 family. Fungal Genet. Biol. 4(5): 542–553 (2008)
Boyce A, Walsh G. Production, purification and application-relevant characterisation of an endo-1,3(4)-β-glucanase from Rhizomucor miehei. Appl. Microbiol. Biotechnol. 7(6): 835–841 (2007)
Murray PG, Grassick A, Laffey CD, Cuffe MM, Higgins T, Savage AV, Planas A, Tuohy MG. Isolation and characterization of a thermostable endo-β-glucanase active on 1,3–1,4-β-D-glucans from the aerobic fungus Talaromyces emersonii CBS 814.70. Enzyme Microb. Technol. 2(9): 90–98 (2001)
Fuchs K-P, Zverlov VV, Velikodvorskaya GA, Lottspeich F, Schwarz WH. Lic16A of Clostridium thermocellum, a noncellulosomal, highly complex endo-β-1,3-glucanase bound to the outer cell surface. Microbiology+ 14(9): 1021–1031 (2003)
Yamamoto M, Ezure T, Watanabe T, Tanaka H, Aono R. C-terminal domain of β-1,3-glucanase H in Bacillus circulans IAM1165 has a role in binding to insoluble β-1,3-glucan. FEBS Lett. 43(3): 41–43 (1998)
Spicer EJF, Goldenthal EI, Ikeda T. A toxicological assessment of curdlan. Food Chem. Toxicol. 3(7): 455–479 (1999)
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Li, J., Zhu, L., Zhan, XB. et al. Purification and characterization of a new endo-β-1,3-glucanase exhibiting a high specificity for curdlan for production of β-1,3-glucan oligosaccharides. Food Sci Biotechnol 23, 799–806 (2014). https://doi.org/10.1007/s10068-014-0108-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10068-014-0108-2