Abstract
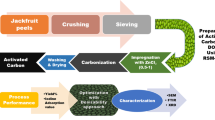
In the adsorption process, the adsorbent structure and its interaction with wastewater are the main determinant of the process efficiency. For this reason, the correct selection of the production conditions for the produced adsorbents is critical. In this study, the reaction time and the concentration of acid, oxidant, substrate, and monomer were selected as independent variables which effect the structure of the polyaniline based composite material. These production variables of polyaniline/beidellite (PANI/BEI) composites were designed by response surface methodology (RSM). According to RSM, 46 different PANI/BEI composites were synthesized and were used as an adsorbent in the removal of acid yellow 194. Thus, the optimum PANI/BEI production conditions were determined. After the identification of the optimum PANI/BEI production conditions, the effect of adsorption conditions such as pH, mixing time, and dye concentration were studied by batch experiments. The maximum adsorption capacity was obtained as 123 (mg g−1) at the T = 25 °C, t = 24 h, pH 3, \({m_s}\) = 0.15 g adsorbent, \({V_s}\) = 50 ml dye solution, and \(\omega\) = 200 rpm. The results presented in this study revealed that the central composite design is a suitable method to optimize the production conditions of PANI/BEI and the prepared PANI/BEI composites has an important potential as an adsorbent for the removal of metal-complex dyes such as AY194. In addition, the polymerization of aniline and the removal of dye were confirmed by different analytical techniques such as BET, XRD, SEM, and EDAX.










Similar content being viewed by others
Abbreviations
- PANI/BEI:
-
Polyaniline/beidellite composite materials
- AY194:
-
Acid yellow 194
- RSM:
-
Response surface methodology
- CCD:
-
Central composite design
- 2FI:
-
Two-factor interaction
- ANOVA:
-
Analyses of variance
- SEM:
-
Scanning electron microscope
References
Raffiea Baseri J, Palanisamy PN, Sivakumar P (2012) Application of polyaniline nano composite for the adsorption of acid dye from aqueous solutions. E-J Chem 9:1266–1275. https://doi.org/10.1155/2012/415234
Qiu B, Xu C, Sun D et al (2014) RSC Advances Polyaniline coating on carbon fiber fabrics for improved hexavalent chromium removal. RSC Adv 4:29855–29865
Khan MA, Dar AM, Arsalan M (2016) Fabrication and characterization of polyaniline based nano-composite with their physico-chemical and environmental applications. J Polym Environ. https://doi.org/10.1007/s10924-016-0850-z
Belaib F, Meniai a H, Lehocine MB (2012) Elimination of phenol by adsorption onto mineral/polyaniline composite solid support. Energy Procedia 18:1254–1260. https://doi.org/10.1016/j.egypro.2012.05.141
Cui H, Qian Y, Li Q et al (2012) Adsorption of aqueous Hg(II) by a polyaniline/attapulgite composite. Chem Eng J 211–212:216–223. https://doi.org/10.1016/j.cej.2012.09.057
Eisazadeh A, Eisazadeh H, Kassim KA (2013) Removal of Pb(II) using polyaniline composites and iron oxide coated natural sand and clay from aqueous solution. Synth Met 171:56–61. https://doi.org/10.1016/j.synthmet.2013.03.014
Janaki V, Vijayaraghavan K, Oh B-TT et al (2012) Starch/polyaniline nanocomposite for enhanced removal of reactive dyes from synthetic effluent. Carbohydr Polym 90:1437–1444. https://doi.org/10.1016/j.carbpol.2012.07.012
Janaki V, Oh B-TT, Shanthi K et al (2012) Polyaniline/chitosan composite: an eco-friendly polymer for enhanced removal of dyes from aqueous solution. Synth Met 162:974–980. https://doi.org/10.1016/j.synthmet.2012.04.015
Javadian H, Vahedian P, Toosi M (2013) Adsorption characteristics of Ni(II) from aqueous solution and industrial wastewater onto polyaniline/HMS nanocomposite powder. Appl Surf Sci 284:13–22. https://doi.org/10.1016/j.apsusc.2013.06.111
Gemeay AH, Mansour I, El-Sharkawy RG, Zaki AB (2005) Preparation and characterization of polyaniline/manganese dioxide composites via oxidative polymerization: effect of acids. Eur Polym J 41:2575–2583. https://doi.org/10.1016/j.eurpolymj.2005.05.030
Gengec E (2015) Color removal from anaerobic/aerobic treatment effluent of bakery yeast wastewater by polyaniline/beidellite composite materials. J Environ Chem Eng 3:2484–2491. https://doi.org/10.1016/j.jece.2015.09.009
Bhadra S, Khastgir D, Singha NK, Lee JH (2009) Progress in preparation, processing and applications of polyaniline. Prog Polym Sci 34:783–810. https://doi.org/10.1016/j.progpolymsci.2009.04.003
Huang W-S, Humphrey BD, MacDiarmid a G (1986) Polyaniline, a novel conducting polymer. J Chem Soc Faraday Trans 82:2385–2400. https://doi.org/10.1039/F19868202385
MacDiarmid AG, Epstein AJ (1989) Polyanilines: a novel class of conducting polymers. Faraday Discuss Chem Soc 88:317. https://doi.org/10.1039/dc9898800317
Ansari R (2006) Application of polyaniline and its composites for adsorption/recovery of chromium(VI) from aqueous solutions. Composites 53:88–94
Ansari R, Mosayebzadeh Z (2011) Application of polyaniline as an efficient and novel adsorbent for azo dyes removal from textile wastewaters. Chem Pap 65:1–8. https://doi.org/10.2478/s11696-010-0083-x
Ghorbani M, Eisazadeh H (2013) Removal of COD, color, anions and heavy metals from cotton textile wastewater by using polyaniline and polypyrrole nanocomposites coated on rice husk ash. Compos B 45:1–7. https://doi.org/10.1016/j.compositesb.2012.09.035
Box GEP, Wilson KB (1951) On the experimental attainment of optimum conditions. J R Stat Soc Ser B 13:1–45
Bezerra MA, Santelli RE, Oliveira EP et al (2008) Response surface methodology (RSM) as a tool for optimization in analytical chemistry. Talanta 76:965–977. https://doi.org/10.1016/j.talanta.2008.05.019
Myers RH, Montgomery DC, Anderson-Cook CM (2009) Response surface methodology: process and product optimization using designed experiments. Wiley, Hoboken
Liu Y, Xiang R (2007) Application of central composite design/response surface methodology in pharmacy experiment design. Chin J Mod Appl Pharm 6:7
Espantaleón AG, Nieto JA, Fernández M, Marsal A (2003) Use of activated clays in the removal of dyes and surfactants from tannery waste waters. Appl Clay Sci 24:105–110. https://doi.org/10.1016/S0169-1317(03)00153-4
Pekey H (2015) Evaluation of electrochemical peroxidation (ECP) process variables for removal of co-complex dye using a central composite design. Desalin Water Treat 57:9845
Anastasi A, Spina F, Prigione V et al (2010) Scale-up of a bioprocess for textile wastewater treatment using Bjerkandera adusta. Bioresour Technol 101:3067–3075. https://doi.org/10.1016/j.biortech.2009.12.067
Taghipour Kolaei Z, Tanzifi M, Yousefi A, Eisazadeh H (2012) Removal of Cd(II) from aqueous solution by using polyaniline/polystyrene nanocomposite. J Vinyl Addit Technol 18:52–56. https://doi.org/10.1002/vnl.20279
Liu X, Zhou W, Qian X et al (2013) Polyaniline/cellulose fiber composite prepared using persulfate as oxidant for Cr(VI)-detoxification. Carbohydr Polym 92:659–661. https://doi.org/10.1016/j.carbpol.2012.09.083
Armes SP, Miller JF (1988) Optimum reaction conditions for the polymerization of aniline in aqueous solution by ammonium persulphate. Synth Met 22:385–393. https://doi.org/10.1016/0379-6779(88)90109-9
Deng J, Wang X, Guo J, Liu P (2014) Effect of the oxidant/monomer ratio and the washing post-treatment on electrochemical properties of conductive polymers. Ind Eng Chem Res 53:13680–13689. https://doi.org/10.1021/ie501366x
Cao Y, Andreatta A, Heeger AJ, Smith P (1989) Influence of chemical polymerization conditions on the properties of polyaniline. Polymer 30:2305–2311. https://doi.org/10.1016/0032-3861(89)90266-8
Gengec E, Ozdemir U, Ozbay B et al (2013) Optimizing dye adsorption onto a waste-derived (modified charcoal ash) adsorbent using box-behnken and central composite design procedures. Water Air Soil Pollut 224:1751. https://doi.org/10.1007/s11270-013-1751-6
Pengthamkeerati P, Satapanajaru T, Singchan O (2008) Sorption of reactive dye from aqueous solution on biomass fly ash. J Hazard Mater 153:1149–1156. https://doi.org/10.1016/j.jhazmat.2007.09.074
Mall ID, Srivastava VC, Agarwal NK (2006) Removal of orange-G and methyl violet dyes by adsorption onto bagasse fly ash—kinetic study and equilibrium isotherm analyses. Dye Pigment 69:210–223. https://doi.org/10.1016/j.dyepig.2005.03.013
Mahanta D, Madras G, Radhakrishnan S, Patil S (2008) Adsorption of sulfonated dyes by polyaniline emeraldine salt and its kinetics. J Phys Chem B 112:10153–10157. https://doi.org/10.1021/jp803903x
Mahanta D, Madras G, Radhakrishnan S, Patil S (2009) Adsorption and Desorption Kinetics of Anionic Dyes on Doped Polyaniline. J Phys Chem B 113(8):2293–2299
Mansour MS, Ossman ME, Farag HA (2011) Removal of Cd(II) ion from waste water by adsorption onto polyaniline coated on sawdust. Desalination 272:301–305. https://doi.org/10.1016/j.desal.2011.01.037
Saha AK, Chaudhuri M (2003) Solar photocatalytic degradation of metal complex azo dyes and treatment of dye house waste. Indian J Eng Mater Sci 10:69–74
Khan AA, Inamuddin (2006) Preparation, physico-chemical characterization, analytical applications and electrical conductivity measurement studies of an “organic-inorganic” composite cation-exchanger: polyaniline Sn(IV) phosphate. React Funct Polym 66:1649–1663. https://doi.org/10.1016/j.reactfunctpolym.2006.06.007
Ansari R, Keivani MB, Delavar AF (2011) Application of polyaniline nanolayer composite for removal of tartrazine dye from aqueous solutions. J Polym Res 18:1931–1939. https://doi.org/10.1007/s10965-011-9600-z
Niwas R, Khan AA, Varshney KG (1999) Synthesis and ion exchange behaviour of polyaniline Sn(IV) arsenophosphate: a polymeric inorganic ion exchanger. Colloids Surf A 150:7–14. https://doi.org/10.1016/S0927-7757(98)00843-7
Öncel MS (2008) Adsorption of copper(II) from aqueous solution by beidellite. Environ Geol 55:1767–1775. https://doi.org/10.1007/s00254-007-1127-6
Mahanta D, Madras G, Radhakrishnan S, Patil S (2008) Adsorption of sulfonated dyes by polyaniline emeraldine salt and its kinetics. J Phys Chem B 112:10153–10157. https://doi.org/10.1021/jp803903x
Qureshi UA, Gubbuk IH, Ersoz M et al (2016) Preparation of polyaniline montmorillonite clay composites for the removal of diethyl hexyl phthalate from aqueous solutions. Sep Sci Technol. https://doi.org/10.1080/01496395.2015.1088029
Acknowledgements
The authors wish to thank The University of Kocaeli for the financial support of this project under contract of BAP 2016/008.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gengec, N.A., Isgoren, M., Kobya, M. et al. Optimization of Beidellite/Polyaniline Production Conditions by Central Composite Design for Removal of Acid Yellow 194. J Polym Environ 26, 2619–2631 (2018). https://doi.org/10.1007/s10924-017-1157-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10924-017-1157-4