Abstract
This present study depicts the successful employment of fixed-bed column for total chromium removal from tannery wastewater in dynamic mode using sodium alginate-powdered marble beads (SA–Marble) as adsorbent. The SA–Marble composite beads prepared were characterized by Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS) and Brunauer, Emmett and Teller (BET) method. The adsorption process performance of this bio-sorbent was examined in batches and columns for real effluent (tannery wastewater). After 90 min, the total chromium removal efficiency could be kept above 90% in the batch experiment. The adsorption kinetics fit better with the pseudo-second-order model, indicating the chemisorption process and the adsorption capacity of about 67.74 mg g−1 at 293 K (C0 = 7100 mg L−1) was obtained. Additionally, dynamic experiments indicate that the total chromium removal efficiency could be maintained above 90% after 120 min at 293 K and 60 min at 318 and 333 K; it’s an endothermic but rapid process. The effects of two adsorption variables (Temperature and time) were investigated using central composite design (CCD), which is a subset of response surface methodology (total Cr, COD, sulfate, and total phosphorus percentage removal). This work paves a new avenue for synthesizing SA–Marble composite beads and provides an adsorption efficiency of total chromium removal from tannery wastewater.
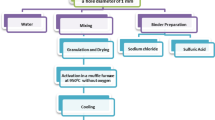
Graphical abstract

Highlights
-
Powder marble coated in sodium-alginate beads (SA–Marble) was prepared & characterized,
-
A reactor with a fixed bed was employed for the dynamic removal of total Cr from tannery wastewater,
-
A capacity of 67.74 mg g−1 at 293 K (C0=7100 mg L-1) allows 90% total Cr removal after 90mn,
-
Pseudo-II-order model simulates well the total Cr endothermic rapid chemisorption process,
-
COD, SO2-4, and TP good removal suggest its use as a successful tannery wastewater treatment.

















Similar content being viewed by others
Data availability
The datasets and materials used and/or analyzed during the current study are available from the corresponding author upon reasonable request.
References
AFNOR, NF T90 023. Dosage des orthophosphates, polyphosphates et phosphore total. Normes nationales et documents normatifs nationaux, ICS 13.060.01 Qualité de l'eau en général, 1990;10.
AFNOR NF T90–101 Détermination de la demande chimique en oxygène, Normes nationales et documents normatifs nationaux, ICS 13.060.50 Détermination des substances chimiques dans l'eau, 2001;7.
Ahmadpoor F, Shojaosadati SA, Mousavi SZ. Magnetic silica coated iron carbide/alginate beads: Synthesis and application for adsorption of Cu (II) from aqueous solutions. Int J Biol Macromol. 2019;128:941–7. https://doi.org/10.1016/j.ijbiomac.2019.01.173.
Aksu Z, Çağatay ŞŞ, Gönen F. Continuous fixed-bed biosorption of reactive dyes by dried Rhizopusarrhizus: determination of column capacity. J Hazard Mater. 2007;143:362–71. https://doi.org/10.1016/j.jhazmat.2006.09.039.
Arshad F, Selvaraj M, Zain J, Banat F, Haija MA. Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep Purif Technol. 2019;209:870–80. https://doi.org/10.1016/j.seppur.2018.06.035.
Aziz F, El Achaby M, Lissaneddine A, Aziz K, Ouazzani N, Mamouni R, Mandi L. Composites with alginate beads: A novel design of nano-adsorbents impregnation for large-scale continuous flow wastewater treatment pilots. Saudi J Biol Sci. 2019. https://doi.org/10.1016/j.sjbs.2019.11.019.
Aziz F, Ouazzani N, Mandi L, Mamoun M, Uheida A. Composite nanofibers of polyacrylonitrile/natural clay for decontamination of water containing Pb (II), Cu (II), Zn (II) and pesticides. Sep Sci Technol. 2017;52:58–70. https://doi.org/10.1080/01496395.2016.1231692.
Bhatnagar A, Sillanpää M, Witek-Krowiak A. Agricultural waste peels as versatile biomass for water purification – a review. Chem Eng J. 2015;270:244–71. https://doi.org/10.1016/j.cej.2015.01.135.
Charumathi D, Das N. Packed bed column studies for the removal of synthetic dyes from textile wastewater using immobilised dead C. tropicalis. Desalination. 2012;285:22–30. https://doi.org/10.1016/j.desal.2011.09.023.
Churio O, Pizarro F, Valenzuela C. Preparation and characterization of iron-alginate beads with some types of iron used in supplementation and fortification strategies. Food Hydrocolloids. 2018;74:1–10. https://doi.org/10.1016/j.foodhyd.2017.07.020.
Da Silva GS, Dos Santos FA, Roth GCLC, Frankenberg. Electroplating for chromium removal from tannery wastewater. Int J Environ Sci Technol. 2019;1–8. https://doi.org/10.1007/s13762-019-02494-110.
Daemi H, Barikani M. Synthesis and characterization of calcium alginate nanoparticles sodium homopolymannuronate salt and its calcium nanoparticles. Sci Iranica. 2012;19(6):2023–8. https://doi.org/10.1016/j.scient.2012.10.005.
Dos Santos CSL, Reis MHM, Cardoso VL, De Resende MM. Electrodialysis for removal of chromium total from effluent: Analysis of concentrated solution saturation. J Environ Chem Eng. 2019;7(5):103380. https://doi.org/10.1016/j.jece.2019.103380.
Elabbas S, Mandi L, Berrekhis F, Pons MN, Leclerc JP, Ouazzani N. Removal of Cr(III) from chrome tanning wastewater by adsorption using two natural carbonaceous materials: eggshell and powdered marble. J Environ Manage. 2016;166:589–95. https://doi.org/10.1016/j.jenvman.2015.11.012.
Elabbas S, Ouazzani N, Mandi L, Berrekhis F, Perdicakis M, Pontviannee S, Pons M-N, Lapicquee F, Leclerc J-P. Treatment of highly concentrated tannery wastewater using electrocoagulation: Influence of the quality of aluminium used for the electrode. J Hazard Mater. 2016;319(2016):69–77. https://doi.org/10.1016/j.jhazmat.2015.12.067.
El-Shafey EI. Behaviour of reduction–sorption of chromium total from an aqueous solution on a modified sorbent from rice husk. Water Air Soil Pollut. 2005;163(1–4):81–102.
Fadali OA, Magdy YH, Daifullah AAM, Ebrahiem EE, Nassar MM. Removal of chromium from tannery effluents by adsorption. J Environ Sci Health, Part A: Toxic/Hazard Subst Environ Eng. 2004;39(2):465–72. https://doi.org/10.1081/ESE-120027537.
Guo Z, Li J, Guo Z, Guo Q, Zhu B. Phosphorus removal from aqueous solution in parent and aluminum-modified eggshells: thermodynamics and kinetics, adsorption mechanism, and diffusion process. Environ Sci Pollut Res. 2017;24(16):14025–36. https://doi.org/10.1007/s11356-017-9072-8.
Hassan AF, Abdel-Mohsen AM, Elhadidy H. Adsorption of arsenic by activated carbon, calcium alginate and their composite beads. Int J Biol Macromol. 2014;68:125–30. https://doi.org/10.1016/j.ijbiomac.2014.04.006.
Haydari I, Lissaneddine A, Aziz K, Ouazzani N, Mandi L, El Ghadraoui A, Aziz F. Optimization of preparation conditions of a novel low-cost natural bio-sorbent from olive pomace and column adsorption processes on the removal of phenolic compounds from olive oil mill wastewater. Environ Sci Pollut Res. 2022;29(53):80044–61.
Ho YS, McKay G. Pseudo-second order model for sorption processes. Process Biochem. 1999;34:401–65. https://doi.org/10.1016/S0032-9592(98)00112-5.
Hu ZH, Omer AM, Ouyang XK, Yu D. Fabrication of carboxylated cellulose nanocrystal/sodium alginate hydrogel beads for adsorption of Pb (II) from aqueous solution. Int J Biol Macromol. 2018;108:149–57. https://doi.org/10.1016/j.ijbiomac.2017.11.171.
Jaafari J, Barzanouni H, Mazloomi S, Farahani NAA, Sharafi K, Soleimani P, Haghighat GA. Effective adsorptive removal of reactive dyes by magnetic chitosan nanoparticles: kinetic, isothermal studies and response surface methodology. Int J Biol Macromol. 2020;164:344–55.
Jaafari J, Ghozikali MG, Azari A, Delkhosh MB, Javid AB, Mohammadi AA, Burakov AE. Adsorption of p-Cresol on Al2O3 coated multi-walled carbon nanotubes: response surface methodology and isotherm study. J Ind Eng Chem. 2018;57:396–404.
Karami A, Karimyan K, Davoodi R, Karimaei M, Sharafie K, Rahimi S, ... Azari A. Application of response surface methodology for statistical analysis, modeling, and optimization of malachite green removal from aqueous solutions by manganese-modified pumice adsorbent. Desalination Water Treatment. 2017;89:150–161.
Khan SU, Islam DT, Farooqi IH, Ayub S, Basheer F. Hexavalent chromium removal in an electrocoagulation column reactor: Process optimization using CCD, adsorption kinetics and pH modulated sludge formation. Process Saf Environ Prot. 2019;122:118–30. https://doi.org/10.1016/j.psep.2018.11.024.
Komoto D, Furuike T, Tamura H. Preparation of polyelectrolyte complex gel of sodium alginate with chitosan using basic solution of chitosan. Int J Biol Macromol. 2019;126:54–9. https://doi.org/10.1016/j.ijbiomac.2018.12.195.
Kuen YL, David JM. Alginate: properties and biomedical applications. Prog Polym Sci. 2012;37:106–26. https://doi.org/10.1016/j.progpolymsci.2011.06.003.
Kumar R, Kim SJ, Kim KH, Lee SH, Park HS, Jeon BH. Removal of hazardous hexavalent chromium from aqueous phase using zirconium oxide-immobilized alginate beads. Appl Geochem. 2018;88:113–21. https://doi.org/10.1016/j.apgeochem.2017.04.002.
Kwak HW, Lee KH. Polyethylenimine-functionalized silk sericin beads for high-performance remediation of hexavalent chromium from aqueous solution. Chemosphere. 2018;207:507–16. https://doi.org/10.1016/j.chemosphere.2018.04.158.
Lagergren S. Zur theorie der sogenannten adsorption geloster stoffe. Kungliga svenska vetenskapsakademiens. Handlingar. 1898;24:1–39.
Lakouraj MM, Mojerlou F, Zare EN. Nanogel and superparamagnetic nanocomposite based on sodium alginate for sorption of heavy metal ions. Carbohyd Polym. 2014;106:34–41. https://doi.org/10.1016/j.carbpol.2014.01.092.
Li H, Li Z, Liu T, Xiao X, Peng Z, Deng L. A novel technology for biosorption and recovery hexavalent chromium in wastewater by bio-functional magnetic beads. Biores Technol. 2018;99(14):6271–9. https://doi.org/10.1016/j.biortech.2007.12.002.
Lissanddine A, Mandi L, El Achaby M, Mousset E, Rene ER, Ouazzani N, Pons MN, Aziz F. Performance and dynamic modeling of a continuously operated pomace olive packed bed for olive mill wastewater treatment and phenol recovery. Chemosphere. 2021;280:130797. https://doi.org/10.1016/j.chemosphere.2021.130797.
Lu J, Li Y, Cong X, Hao Y, Wang C. Influence of different reinforcements on toughening and strengthening of sintered stoneware from modified marble powder. Constr Build Mater. 2018;159:99–106. https://doi.org/10.1016/j.conbuildmat.2017.10.099.
Lv X, Zhang Y, Fu W, Cao J, Zhang J, Ma H, Jiang G. Zero-valent iron nanoparticles embedded into reduced graphene oxide-alginate beads for efficient chromium total removal. J Colloid Interface Sci. 2017;506:633–43. https://doi.org/10.1016/j.jcis.2017.07.024.
Mahdavinia GR, Mousanezhad S, Hosseinzadeh H, Darvishi F, Sabzi M. Magnetic hydrogel beads based on PVA/sodium alginate/laponite RD and studying their BSA adsorption. Carbohyd Polym. 2016;147:379–91. https://doi.org/10.1016/j.carbpol.2016.04.024.
Mahmood Z, Amin A, Zafar U, Raza MA, Hafeez I, Akram A. Adsorption studies of cadmium ions on alginate–calcium carbonate composite beads. Appl Water Sci. 2017;7(2):915–21. https://doi.org/10.1007/s13201-015-0302-2.
Mahmood Z, Nasir S, Jamil N, Sheikh A, Akram A. Adsorption studies of phosphate ions on alginate-calcium carbonate composite beads. Afr J Environ Sci Technol. 2015;9(3):274–81. https://doi.org/10.5897/AJEST2014.1784.
Mandi L, Tiglyene S, Jaouad A. Depuration of tannery effluent by phytoremediation and infiltration percolation under arid climate. Options Mediterraneennes. 2009;88:199–205.
Mohammadi AA, Dehghani MH, Mesdaghinia A, Yaghmaian K, Es’haghi Z. Adsorptive removal of endocrine disrupting compounds from aqueous solutions using magnetic multi-wall carbon nanotubes modified with chitosan biopolymer based on response surface methodology: Functionalization, kinetics, and isotherms studies. Int J Biol Macromol. 2020;155:1019–29.
Mohammed K, Sahu O. Recovery of chromium from tannery industry waste water by membrane separation technology: health and engineering aspects. Scientific African. 2019;4:e00096. https://doi.org/10.1016/j.sciaf.2019.e00096.
Mohammed N, Grishkewich N, Waeijen HA, Berry RM, Tam KC. Continuous flow adsorption of methylene blue by cellulose nanocrystal-alginate hydrogel beads in fixed bed columns. Carbohyd Polym. 2016;136:1194–202. https://doi.org/10.1016/j.carbpol.2015.09.099.
Mohan D, Singh KP, Singh VK. Removal of hexavalent chromium from aqueous solution using low-cost activated carbons derived from agricultural waste materials and activated carbon fabric cloth. Ind Eng Chem Res. 2005;44(4):1027–42. https://doi.org/10.1021/ie0400898.
Obeid L, El Kolli N, Dali N, Talbot D, Abramson S, Welschbillig M, Bée A. Adsorption of a cationic surfactant by a magsorbent based on magnetic alginate beads. J Colloid Interface Sci. 2014;432:182–9. https://doi.org/10.1016/j.jcis.2014.06.027.
Oladipo AA, Adeleye OJ, Oladipo AS, Aleshinloye AO. Bio-derived MgO nanopowders for BOD and COD reduction from tannery wastewater. J Water Process Eng. 2017;16:142–8. https://doi.org/10.1016/j.jwpe.2017.01.003.
Omer AM, Khalifa RE, Hu Z, Zhang H, Liu C, Ouyang XK. Fabrication of tetraethylenepentamine functionalized alginate beads for adsorptive removal of total chromium from aqueous solutions. Int J Biol Macromol. 2018;125:1221–31. https://doi.org/10.1016/j.ijbiomac.2018.09.097.
Park HJ, Jeong SW, Yang JK, Kim BG, Lee SM. Removal of heavy metals using waste eggshell. J Environ Sci. 2007;19(12):1436–41. https://doi.org/10.1016/S1001-0742(07)60234-4.
Peer FE, Bahramifar N, Younesi H. Removal of Cd (II), Pb (II) and Cu (II) ions from aqueous solution by polyamidoamine dendrimer grafted magnetic graphene oxide nanosheets. J Taiwan Inst Chem Eng. 2018;87(2018):225–40. https://doi.org/10.1016/j.jtice.2018.03.039.
Periyasamy S, Gopalakannan V, Viswanathan N. Hydrothermal assisted magnetic nano-hydroxyapatite encapsulated alginate beads for efficient total chromium uptake from water. J Environ Chem Eng. 2018;6(1):1443–1404. https://doi.org/10.1016/j.jece.2018.01.007.
Rassis DK, Saguy IS, Nussinovitch A. Collapse, shrinkage and structural changes in dried alginate gels containing fillers. Food Hydrocolloids. 2002;16:139–51. https://doi.org/10.1016/S0268-005X(01)00071-6.
Sahraei R, Pour ZS, Ghaemy M. Novel magnetic bio-sorbent hydrogel beads based on modified gum tragacanth/graphene oxide: Removal of heavy metals and dyes from water. J Clean Prod. 2017;142:2973–84. https://doi.org/10.1016/j.jclepro.2016.10.170.
Sallam A, Al-Zahrani M, Al-Wabel M, Al-Farraj A, Usman A. Removal of Total chromium and Toxic Ions from Aqueous Solutions and Tannery Wastewater Using Polymer-Clay Composites. Sustainability. 2017;9(11):1993. https://doi.org/10.3390/su9111993.
Selvaraj H, Aravind P, Sundaram M. Four compartment mono selective electrodialysis for separation of sodium formate from industry wastewater. Chem Eng J. 2018;333:162–9. https://doi.org/10.1016/j.cej.2017.09.150.
Sharafi K, Pirsaheb M, Gupta VK, Agarwal S, Moradi M, Vasseghian Y, Dragoi EN. Phenol adsorption on scoria stone as adsorbent-Application of response surface method and artificial neural networks. J Mol Liq. 2019;274:699–714.
Soleimani H, Mahvi AH, Yaghmaeian K, Abbasnia A, Sharafi K, Alimohammadi M, Zamanzadeh M. Effect of modification by five different acids on pumice stone as natural and low-cost adsorbent for removal of humic acid from aqueous solutions-Application of response surface methodology. J Mol Liq. 2019;290:111181.
Sutananta W, Craig DQ, Newton JM. An evaluation of the mechanisms of drug release from glyceride bases. J Pharm Pharmacol. 1995;47(3):182–7. https://doi.org/10.1111/j.2042-7158.1995.tb05775.x.
Tiglyene S, Jaouad A, Mandi L. Treatment of tannery wastewater by infiltration percolation: chromium removal and speciation in soil. Environ Technol. 2008;29:613–24. https://doi.org/10.1080/09593330801983888.
Torit J, Phihusut D. Phosphorus removal from wastewater using eggshell ash. Environ Sci Pollut Res. 2018;1–9. https://doi.org/10.1007/s11356-018-3305-3.
Vazifehkhoran AH, Shin SG, Triolo JM. Use of tannery wastewater as an alternative substrate and a pre-treatment medium for biogas production. Biores Technol. 2018;258:64–9. https://doi.org/10.1016/j.biortech.2018.02.116.
Vipin AK, Ling S, Fugetsu B. Removal of Cs+ and Sr2+ from water using MWCNT reinforced Zeolite-A beads. Microporous Mesoporous Mater. 2016;224:84–8. https://doi.org/10.1016/j.micromeso.2015.11.024.
Xiao S, Zhao L, Leng X, Lang X, Lian J. Synthesis of amorphous TiO2 modified ZnO nanorod film with enhanced photocatalytic properties. Appl Surf Sci. 2014;299(2014):97–104. https://doi.org/10.1016/j.apsusc.2014.01.192.
Xu S, Niu X, Hou Z, Gao C, Lu J, Pang Y, Li T. A multifunctional gelatine–quaternary ammonium copolymer: An efficient material for reducing dye emission in leather tanning process by superior anionic dye adsorption. J Hazard Mater. 2019;121142. https://doi.org/10.1016/j.jhazmat.2019.121142.
Yan Y, An Q, Xiao Z, Zheng W, Zhai S. Flexible core-shell/bead-like alginate@ PEI with exceptional adsorption capacity, recycling performance toward batch and column sorption of Cr total. Chem Eng J. 2017;313:475–86. https://doi.org/10.1016/j.cej.2016.12.099.
Yousefi M, Nabizadeh R, Alimohammadi M, Mohammadi AA, Mahvi AH. Removal of phosphate from aqueous solutions using granular ferric hydroxide process optimization by response surface methodology. Desalin Water Treat. 2019;158:290–300.
Zhang K, Batterman S. Air pollution and health risks due to vehicle traffic. Sci Total Environ. 2013;400:307–16. https://doi.org/10.1016/j.scitotenv.2013.01.074.
Zrid R, Bentiss F, Ali RAB, Belattmania Z, Zarrouk A, Elatouani S, Sabour B. Potential uses of the brown seaweed Cystoseira humilis biomass: 1-Sodium alginate yield, FT-IR, H NMR and rheological analyses. J Mater. 2016;7(2):613–20.
Funding
This work was supported by the Morocco-Tunisian bilateral scientific cooperation project (20/MT-PRD-02).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval and consent to participate
We all declare that manuscript reporting studies do not involve any human participants, human data, or human tissue. So it is not applicable.
Consent for publication
Our manuscript does not contain data from any individual person, so it is “Not applicable.”
Competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have influenced the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Lissaneddine, A., Aziz, K., Ouazzani, N. et al. Continuous treatment of highly concentrated tannery wastewater using novel porous composite beads: Central composite design optimization study. J Environ Health Sci Engineer 21, 513–532 (2023). https://doi.org/10.1007/s40201-023-00878-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40201-023-00878-7