Abstract
To describe the principles and the first clinical application of a novel prototype automated weaning system called Evita Weaning System (EWS). EWS allows an automated control of all ventilator settings in pressure controlled and pressure support mode with the aim of decreasing the respiratory load of mechanical ventilation. Respiratory load takes inspired fraction of oxygen, positive end-expiratory pressure, pressure amplitude and spontaneous breathing activity into account. Spontaneous breathing activity is assessed by the number of controlled breaths needed to maintain a predefined respiratory rate. EWS was implemented as a knowledge- and model-based system that autonomously and remotely controlled a mechanical ventilator (Evita 4, Dräger Medical, Lübeck, Germany). In a selected case study (n = 19 patients), ventilator settings chosen by the responsible physician were compared with the settings 10 min after the start of EWS and at the end of the study session. Neither unsafe ventilator settings nor failure of the system occurred. All patients were successfully transferred from controlled ventilation to assisted spontaneous breathing in a mean time of 37 ± 17 min (± SD). Early settings applied by the EWS did not significantly differ from the initial settings, except for the fraction of oxygen in inspired gas. During the later course, EWS significantly modified most of the ventilator settings and reduced the imposed respiratory load. A novel prototype automated weaning system was successfully developed. The first clinical application of EWS revealed that its operation was stable, safe ventilator settings were defined and the respiratory load of mechanical ventilation was decreased.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
Despite being lifesaving in critical clinical situations it has been recognized that mechanical ventilation can induce lung injury [1] and diaphragmatic dysfunction [2]. Therefore the primary aim of a ventilation strategy should be to decrease the overall ventilation time. Several studies revealed a significantly shorter overall ventilation time in protocol-guided weaning when compared to usual care [3–13]. Therefore, the use of a weaning protocol is recommended [14, 15].
One might suggest that not only the implemented weaning protocol or the applied ventilation mode but the whole strategy of ventilator therapy influences ventilation time, weaning time and outcome. Basically there exist two different strategies: a classical and an alternative one. Classically, the ventilator therapy secures normal alveolar ventilation and adequate oxygenation of the patient. The weaning process is initiated after the reasons for prolonged ventilation have been ruled out [15]. Depending on the clinical condition of the patient, spontaneous breathing trials are then undertaken.
In contrast, an alternative strategy prefers a smooth transition to the weaning phase at the early beginning of the ventilator therapy. Taking into account that mechanical ventilation itself leads to lung injury, the major target of this strategy is to decrease the respiratory load imposed by mechanical ventilation as soon as possible, thereby minimizing the ventilator induced lung injury and reducing ventilation time. The basic principles of this strategy are [16–20]:
-
Guarantee oxygenation of the patient at the lowest possible inspired fraction of oxygen (FIO2).
-
Assess oxygenation performance and find an optimal level of positive end-expiratory pressure (PEEP).
-
Use the lowest possible level of inspiratory pressure (Pinsp) and pressure support (PS) to reach normal alveolar ventilation.
-
Adapt inspiration (TI) and expiration times (TE) to the actual breathing mechanics and thereby avoid intrinsic PEEP.
-
Support assisted spontaneous breathing by decreasing mechanical breathing frequency (fmech) immediately when spontaneous breathing activity occurs.
The principle idea behind this strategy is that oxygenation, alveolar ventilation and spontaneous breathing activity can be treated rather independently. Thus, weaning may be possible in one or two of the aspects, whereas the support of the ventilator is still high in the third aspect.
It is apparent that this weaning strategy requires continuous monitoring of patients by well-trained and motivated members of the staff. The ventilator settings need to be adjusted several times per hour to assure an optimal matching between the patient’s needs, the ventilator performance and the strategy outlined above. An automated system able to continually monitor the patient and adjust the ventilator settings according to a protocol could be a reasonable solution.
In this paper, we describe a novel prototype knowledge- and model-based system for automated weaning of patients from mechanical ventilation called Evita Weaning System (EWS). This system incorporates the already outlined alternative weaning strategy. In a selected case study we tested the hypotheses that EWS is able (1) to transfer the patients from controlled mechanical ventilation to assisted spontaneous breathing and (2) to decrease the respiratory load of the patients. The respiratory load is measured by a newly developed scoring system.
2 Materials and methods
2.1 Description of the system
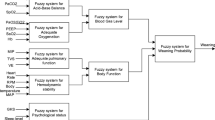
EWS operates with a modified version of a commercially available intensive care respirator Evita 4 (Dräger Medical GmbH, Lübeck, Germany) with optional modules for measuring peripheral oxygen saturation (SpO2), the end-tidal partial pressure of CO2 (PE′CO2) and a serial communication port for remote respirator control (Fig. 1). The software of the ventilator (version 2.98) was modified by the manufacturer (Dräger Medical GmbH, Lübeck, Germany) to enable remote control of the respirator (except for FIO2) by an external computer. The expert knowledge about mechanical ventilation and weaning is encoded in a knowledge base (KB) while the actual respiratory physiology (i.e. current gas exchange and lung mechanics) is described by a basic physiological lung model.
EWS is designed to provide automated control in the biphasic positive airway pressure/assisted spontaneous breathing (BIPAP/ASB) mode. FIO2 was not controlled automatically. However, EWS proposed novel settings for FIO2 (according to Table E4) which were then manually set by the user. The primary aim of EWS is to decrease respiratory load which is defined as the level of support provided by the ventilator to reach basic therapeutic goals. The current respiratory load is determined with the help of a newly developed scoring system (Table 1). To decrease the respiratory load, the system uses the following hypotheses:
-
Is it possible to decrease FIO2?
-
Is it possible to decrease PEEP when oxygenation performance is good/sufficient or to increase PEEP when oxygenation performance is moderate or bad?
-
Is it possible to decrease Pinsp and PS?
-
Is it possible to induce assisted spontaneous breathing when no spontaneous breathing activity was detected during the last 3 h?
The system tests these hypotheses sequentially. The target range for arterial partial pressure of oxygen (PaO2) is 80 to 100 mm Hg and the proposed setting for FIO2 is determined according to supplementary Table E4. Automated PEEP trials are performed as described in the electronic supplementary material. A systematical approach is used to decrease Pinsp, PS and fmech. Depending on the measured respiratory time constant, patients are classified into one of the three available categories (normal, obstructive, restrictive). The value of the minute ventilation (MV) to achieve a target arterial partial pressure of carbon dioxide (PaCO2) is transferred to the EWS by the lung model after calibration or adjusted according to the ideal body weight if no calibrated lung model is available. Mechanical breathing frequency and tidal volume (VT) are derived subsequently (see electronic supplementary material sections 1.6.1. and 1.6.2). Monitoring is performed by the continuous measurement of SpO2 and PE′CO2.
KB requires the input of PaO2, PaCO2 and the desired MV. These values are provided by the physiological lung model. Before the model is able to provide these values it has to be aligned to the patient’s respiratory status. This task is performed by a calibration which is initiated automatically after the start-up of the system. After the calibration, the model displays the simulated blood gas values and the internal model parameters (e.g. fractions of shunt and deadspace). The user can then accept the model calibration or provide measured or estimated arterial blood gas values to the model. In the latter case, the calibration process is reinitiated and the results are displayed again to the user. After acceptance, the calibration process is finished. To calibrate the model to lung healthy patients, the physician can use a standard configuration of the model (i.e. standard shunt and deadspace fractions of 5 and 10 %, respectively). In this case, no arterial blood gas values are needed.
As common clinical practice, the physician can define a target PaCO2 value with a default of 40 mm Hg. Then, the model calculates the MV that is required to achieve the target PaCO2. This MV is defined as target MV (MVtarget). When the user accepts the model calibration, MVtarget is once determined by three simulations with different MV and PaCO2 values. With these three paired measurements, a parabola can sufficiently be described and the needed MV can be assessed. This MVtarget is then applied to the patient.
Then, the model simulates PaO2 and PaCO2 values continuously and its validity is checked with the measured SpO2 and PE′CO2. When a drift between the model and the patient is detected, a recalibration with actual blood gas values is needed. After a successful recalibration, MVtarget is recalculated. Furthermore, the user has the opportunity to initiate a recalibration of the lung model at any time. To calibrate the model to lung healthy patients, the physician can use a standard configuration of the model. In this case, no arterial blood gas values are needed.
A detailed description of the physiological lung model and KB is given in the electronic supplementary material.
2.2 Study protocol
The selected case study was accomplished at the intensive care unit (ICU) of the Department of Anesthesiology and Intensive Care Medicine, University Medical Center Schleswig–Holstein, Campus Kiel. The study was approved by the local ethics committee. 19 postsurgical patients were included into the study when fulfilling the following inclusion criteria: written informed consent given by the patient or the next of kin, BIPAP/ASB ventilator mode via an artificial airway with no spontaneous breathing activity, reduction of the respiratory load considered possible by the physician in charge, patient monitoring established (electrocardiogram, SpO2, arterial line). Exclusion criteria were: high-dose catecholamines, anticipated surgical complication, systemic infection, pregnancy. Detailed patients’ characteristics are given in Table 2. Propofol and sufentanil were continuously infused to sustain analgesia and sedation. All patients were connected to the study ventilator and the ventilator settings set in the ICU were maintained. The used alarm settings are shown in Table 3. The flow trigger was set to 5 l min−1, the pressure slope was set to 0.2 s. The upper limit of PEEP was 15 cm H2O, the lower limit was 3 cm H2O.
After verifying the stable status of the patient, the EWS was started (start of the study period). The system needed 10 to 30 min for calibrating the model. To get the best possible adaptation between model and patient, the measured blood gas values were entered. At the end of this process the system displayed the simulated distribution of the three compartments of the lung (normal, dead space, shunt) as well as the actual simulated blood gas values. Then, EWS began to adopt the ventilator settings automatically. The weaning period was initiated by decreasing the level of the propofol infusion (2 ml h−1 every 10 min) until the patient recovered to a Ramsay Score [21] of two to three and by switching the operating mode of the EWS to weaning. Weaning was then accomplished by the automated weaning system. Automated weaning was stopped either when patients were extubated or when no significant changes of the automatic ventilator therapy were suggested. To assure patient’s safety, all changes of the ventilator settings were supervised by an experienced physician who was able to stop the system anytime.
2.3 Data acquisition and analysis
All Evita 4 settings and measurements as well as the results of the KB evaluations and the model simulations were automatically acquired and saved in a database. Rejections of the EWS settings by an experienced physician were documented separately. For safety analysis, all breath-by-breath ventilator settings and measurements with defined alarm limits (Table 3) were recorded and checked. The EWS performance was assessed by comparing the ventilator settings, PE′CO2, PaO2 divided by FIO2 (whereby PaO2 was estimated from SpO2 according to Table 4) and the respiratory load score acquired (1) at the onset of the EWS operation (original physician’s settings), (2) 10 min after the start of automated ventilation (initial EWS settings) and (3) by the end of the study period (final EWS settings). To determine the time required for achieving sufficient assisted spontaneous breathing, the time interval between the first spontaneous breath and the permanent reduction of mechanical ventilation (<3 mechanical breaths per minute) was measured. ANOVA for repeated measurements and Bonferroni post-test for multiple comparisons were used for statistical analysis. Statistical significance was accepted at p values <0.05.
3 Results
The ventilator settings and measurements acquired at the three time points (as defined in the Sect. 2) during the study are shown in Table 5. The changes of the ventilator settings accomplished by EWS after 10 min of operation resulted in a significant decrease in FIO2 when compared with the initial settings ordered by the responsible physician. At the end of the study period, a significant decrease in FIO2, Pinsp, PS, mean airway pressure (Pmean) and VT as well as a significant increase in PE′CO2 were noted. The mean score of respiratory load fell significantly from 3.9 ± 0.3 and 3.8 ± 0.5 to 3.0 ± 0.8 at the three measurement phases followed. The high respiratory load during the first two measurements was caused by the non-existent spontaneous breathing activity whereas, during the final settings, it was mainly influenced by PEEP. More detailed data of the respiratory load sub scores are given in Table 5. All patients could be transferred from controlled ventilation to assisted spontaneous breathing. The corresponding mean time interval was 37 ± 17 min. Consequently, fmech was lower and spontaneous breathing frequency (fspont) was higher than during preceding measurement periods.
All patients were orally intubated except for patient 5 (tracheal cannula). The mean (±SD) time a patient spent on the automated weaning system was 173 ± 53 min. All patients tolerated the ventilation with the EWS well. No interruption was necessary and the responsible physician classified all ventilator settings proposed by the EWS as clinically acceptable. No technical defects occurred, however, a restart of the external computer was necessary in one patient. An overview of the minimal, maximal, and median ventilator parameters is shown in Table 6.
Safe ventilator settings were achieved in 97.2 % of the total automatic ventilation time (Fig. 2). The following safety violations occurred. The upper safety limit for VT was exceeded in patients 5, 8, 12, 15 and 19 with a maximum Pinsp of 29 cm H2O during these episodes. In patient 5, assisted spontaneous breathing was present with the least possible PS of 5 cm H2O. Therefore, the system could not further influence VT. In patients 8, 15 and 19, the EWS immediately reduced PS. In patient 12 the endotracheal tube was obstructed, therefore, the system increased the inspiratory pressures. After the removal of obstruction, the inspiratory pressures were automatically decreased by the EWS. The lower safety limit for MV was violated in patients 13, 16 and 19 for the following reasons: (1) running test to initiate spontaneous breathing (immediately canceled by the system), (2) coughing or squeezing the endotracheal tube during the first spontaneous breaths. The system did not change the ventilator parameters in most cases, in patient 16, the inspiratory pressure was increased by 2 cm H2O. PE′CO2 fell twice below the lower safety limit in patient 4 who suffered from a previously unknown pulmonary embolism.
A total of 39 PEEP trials were accomplished. Out of these, 15 tests were completed and the remaining 24 were interrupted due to instable FIO2 or MV. As a result of the 15 completed trials, PEEP was decreased in ten, remained unchanged in four and increased in one case.
Fifteen patients out of the total of 19 patients studied were weaned successfully with EWS and were extubated at the end of the study period.
4 Discussion
We described a knowledge- and model-based system for automated weaning of patients from mechanical ventilation and its first clinical application in a selected case study. Our system was able to transfer all patients automatically from controlled to assisted mechanical ventilation in a short period of time. Moreover, the respiratory load was decreased significantly by EWS.
4.1 Performance of the system
Performance of the system was assessed by a newly developed scoring system because no validated scoring system was available at the time of the study. The goal of the scoring system was to classify the respiratory load imposed on the lungs by the respirator and to monitor its time course. The assignment of the points used in the sub scores is derived from the basic principles of lung physiology and clinical experience with mechanical ventilation. The higher FIO2, PEEP and the pressure amplitude the higher the respiratory load score and vice versa. The lower the spontaneous breathing activity the higher respiratory load score and vice versa. However, higher PEEP may also be interpreted as lower respiratory load given because it leads to a larger amount of recruited lung tissue. We did not introduce this phenomenon into our system as EWS was not capable of detecting the amount of the recruited lung tissue. After successful weaning of 19 patients with this system, it can be concluded that the assignment of the points was appropriate. The decrease in respiratory load, particularly explained by an increased spontaneous breathing activity, was detected by the score. However, patients could be successfully extubated even with a high respiratory load score. For instance, patient 3 exhibited a high respiratory load score because PEEP was 5 cm H2O and FIO2 was 0.35. Therefore, the limits for the respiratory load score have to be adapted if this score is to be applied as an extubation criterion in the future.
4.2 Safety
In this selected case study, EWS determined safe ventilator settings. VT values beyond the upper safety limits were properly identified and KB immediately lowered the inspiratory pressures. The inspiratory pressures during these phases were in an acceptable range. The violation of the lower safety limit for PE’CO2 was observed in a patient with a previously unknown pulmonary embolism. The pathologically increased dead space ventilation was detected by the model and the MVtarget was appropriately increased. The violation of the lower safety limits for MV was not critical as it was induced by the concept to challenge spontaneous breathing.
Median SpO2 of 99 % was observed in all patients and SpO2 was always higher than 93 % except for two patients. In patient 10, FIO2 was immediately increased from 0.4 to 0.5, in patient 18 a false low value occurred. Hence, EWS provided safe oxygenation in the studied patients [22]. However, it has to be mentioned that EWS only proposed novel settings for FIO2. For safety reasons, these settings were transferred manually to the ventilator. PEEP showed a slight but statistically not significant tendency towards higher values at the beginning and reached relatively normal values by the end of the study.
Normal PE′CO2 values were determined in all patients during the study period except for patient 4 suffering from the already mentioned pulmonary embolism. The abnormal difference between the PE′CO2 and PaCO2 was the first diagnostic marker. This patient is a good example for the inadequacy of PE′CO2 as a criterion for controlling alveolar ventilation, as already discussed by Brunner [23], and circumstantiates the advantage of using a physiological lung model in automated ventilation. After the adjustment of the model to the patient’s lung status, the dead space compartment rose to 71 %. This means, that the pathologic situation was adequately reproduced by the model. The determined MVtarget was 13 l min−1. After the adjustment of ventilator settings according to the EWS proposal, PaCO2 normalized.
4.3 Comparison between EWS and physician
The ventilator settings and measurements acquired 10 min after the start of the automatic ventilation reflected the initial changes performed by EWS. A decrease in inspiratory pressure was proposed by EWS in patients with slight hyperventilation with a consecutive decrease in VT and MV. EWS also reduced FIO2. In patients with spontaneous breathing activity, EWS decreased fmech, otherwise the system applied fmech depending on the actual breathing mechanics. Most of the patients were ventilated with a higher fmech and a lower VT than the values set by the physician. Although EWS was not programmed with the primary aim of ventilating the patient with low VT, the results indicate that lung protective ventilation with low VT was achieved in our patients. This is in agreement with the findings of the acute respiratory distress syndrome network showing the negative effect of mechanical ventilation with high tidal volumes [24]. At the end of the study period, EWS could successfully wean all patients as reflected by a decrease in respiratory load score.
4.4 PEEP trials
In the present study the therapeutic efficacy of the conducted PEEP trials could not be proved. PEEP showed no significant changes over the whole study period because the trials were performed only hourly and the patients were ventilated for a relatively short period of time. Nevertheless, successful PEEP trials with subsequent PEEP changes were performed in some cases. These changes were adequate according to our clinical assessment.
4.5 Technical limitations
Two technical limitations were found. The communication between the respirator and PC was too slow. Consequently, when a patient breathed at a rate higher than 25 breaths min−1, the system could not identify all breaths. Another limitation was related to the performance of the physiological lung model. When a patient began to breathe spontaneously the respiratory mechanics frequently changed. Under these circumstances, the model could not adequately map the patient in the short period of time available and the simulated blood gases were not reliable. However, EWS checked the validity of the simulated blood gases and if the values were classified as not valid they were not used. Even in such cases EWS was able to determine adequate ventilatory settings and continued the automated ventilation of the patient using either the previously determined MV or MV selected by the user.
4.6 Implemented strategy
We acknowledge that our electronically implemented weaning strategy may arise some questions. Therefore, we present the empiric or scientific rationales for each of the basic therapeutic goals of the system below:
-
Guarantee oxygenation of the patient at the lowest possible FIO2
Albeit no evidence-based recommendation for an optimum FIO2 setting is available, we implemented this strategy into our system for the following reasons: (1) To minimize the risk of oxygen toxicity while securing sufficient oxygenation [25]. (2) To decrease FIO2 which is an established clinical process during weaning. An overview of the existing automated strategies is given in [26].
-
Assess oxygenation performance and find an optimal level of PEEP.
The current weight of literature is indifferent regarding the optimum level of PEEP [17, 27–29]. In patients with acute respiratory distress syndrome, two studies revealed that setting of PEEP above the lower inflection point improved outcome [30, 31]. However, a low-flow-pressure-volume-maneuver should not be executed automatically by an automatic weaning system without careful monitoring of the patient. A reasonable solution for the clinical practice (and for EWS) may be the titration of PEEP in patients with impaired oxygenation performance. PEEP reflects the disease state of the ventilated lung. Since this disease state of the lung does not change very quickly, it is not recommended to change PEEP frequently.
-
Use the lowest Pinsp and PS to reach normal alveolar ventilation
With the help of this therapeutic goal, we aim at decreasing the ventilatory support as soon as possible. Consequently, weaning from mechanical ventilation could be accelerated, which may lead to a shorter overall ventilation time.
-
Adapt TI and TE to the actual breathing mechanics and thereby avoid intrinsic PEEP.
An incomplete expiration avoids derecruitment of the lungs which may lead to an improved oxygenation [32]. However, the amount of the generated intrinsic PEEP is not predictable and this could lead to an unwanted decrease in alveolar ventilation. Therefore, it appears to be safer to guarantee complete expiration and use extrinsic PEEP instead of the less controllable intrinsic PEEP. A further advantage of this approach is the prolongation of the inspiratory time to its maximum possible value for a preset I:E-ratio which in turn allows the application of low inspiratory pressures.
-
Use the ventilation mode BIPAP/ASB.
-
Support assisted spontaneous breathing by using BIPAP/ASB and by decreasing fmech immediately when spontaneous breathing activity occurs.
When the patient does not breathe spontaneously, BIPAP/ASB is equal to pressure controlled ventilation (PCV). Spontaneous breathing is supported in this mode in three different ways. First, the patient can breathe spontaneously on both pressure levels without additional support of the respirator. Second, the patient can trigger a mechanical breath and third, the patient can trigger additional pressure support breaths on the lower pressure level. Early support of spontaneous breathing compared to conventional mechanical ventilation was associated with lower peak inspiratory pressures [33, 34], improvement of ventilation-perfusion ratio [35], improvement of splanchnicus perfusion [36], and less need of sedative drugs [34, 37]. One randomized controlled trial in patients with acute lung injury showed a significantly lower total ventilation time, lower length of ICU and hospital stay in patients who were allowed to breathe spontaneously at an early point of time [37]. Therefore, supporting spontaneous breathing with the used ventilatory mode and the implemented strategy is reasonable.
4.7 Comparison with other systems
Automated control of mechanical ventilation has been intensely studied with more than 50 publications from 1953 until to date. Many systems were already designed to control fmech [38–48]. An excellent overview is given in [26]. Also, the combination of physiological lung models and rule-based systems were used by several authors previously [49–51]. In 1993 Rutledge et al. [51] published an automated system (VENTPLAN) for the control of four ventilatory settings (FIO2, PEEP, VT, and fmech). Comparable to EWS the authors used a combination of a mathematical modeling module (based on the physiological lung model by Riley and Cournand [52]) and a plan evaluator for the control of the ventilatory settings. VENTPLAN used the model not only to map the current respiratory status of the patient. It was also applied to predict the patients’ respiratory status according to the planned therapy. To our knowledge, this system was only evaluated in a retrospective study [51]. Rees et al. [50] published a decision support system that combined physiological models with a penalty system. The models could be tuned to an individual patient and with the help of the modeled parameters the system supported the clinician in interpreting the data into a clinical representation of the patient. Finally, the system proposed a ventilatory strategy that resulted in a minimum total penalty (indicating a high clinical preference favoring the strategy). The systems by Rees et al. and Ruthledge et al. determined proper ventilatory settings. However, neither of them was designed to follow a weaning strategy. Our system aims at decreasing the respiratory load and at weaning the patient from mechanical ventilation.
To date, three automated weaning systems are commercially available: SmartCare/PS (Dräger Medical, Lübeck, Germany), Adaptive Support Ventilation (Hamilton Medical, Bonaduz, Switzerland) and Intellivent-ASV (Hamilton Medical, Bonaduz, Switzerland).
SmartCare/PS is the commercially available version of NéoGanesh, which was developed by Brochard et al. [53]. The system controls PS in the pressure support mode (PSV) with the aim of keeping the patient in a zone of respiratory comfort (ZORC) [54]. It has been shown that SmartCare/PS is able to keep the patients in ZORC [53, 55] because it adjusts the settings of PS significantly more often than a physician [55]. SmartCare/PS is a practicable predictor for a successful extubation trial [56] and was intensely studied in several randomized controlled trials [57–59]. In one multicenter randomized controlled trial the system decreased ventilation time significantly when compared to usual care [57]. However, the performance of SmartCare/PS is limited as it is designed to work only in the PSV mode and solely the PS level is automatically controlled.
Adaptive support ventilation (ASV) is a combined PCV/PSV mode with automatic control of inspiratory pressures and fmech and is aimed mainly at decreasing the patients’ work of breathing [60–64]. ASV works well in anaesthetized and paralyzed patients with obstructive and restrictive lung diseases [65] and improves the patient-ventilator interaction [66]. Results concerning the time needed for weaning patients from mechanical ventilation are ambiguous [67–70]. ASV stands as a milestone in automated control of mechanical ventilation and its performance has been checked under clinical conditions. However, a fully automated ventilation of a patient is not possible with ASV.
Intellivent-ASV is a further development of ASV and is the first commercially available system that provides an automated control of all ventilatory settings. The efficacy and safety of Intellivent-ASV was compared to ASV in one randomized crossover study in 50 ventilated patients without spontaneous breathing activity [71]. The authors concluded that Intellivent-ASV was safe and able to ventilate patients with lower Pinsp and FIO2. The results of this first clinical trial look promising and further studies are ongoing (clinicaltrials.gov ID NCT01577667, NCT01489085).
In comparison to the above mentioned systems, EWS is able to set up all ventilatory settings automatically except for FIO2 and it was evaluated in the present study. We were able to transfer the medical knowledge about mechanical ventilation therapy into a knowledge-based system. We further introduced the basic physiological lung model to map the clinical picture of the patient in our system. In this study, we were able to show that this combination provided safe ventilatory settings and decreased the respiratory load. However, since no randomized controlled trial comparing two or more of the available automated weaning systems has ever been conducted it is still unclear which system performs the best.
5 Conclusions
A novel knowledge- and model-based prototype system for automated weaning provided a soft transfer from controlled to assisted ventilation and succeeded in decreasing the respiratory load of mechanical ventilation. The combination of a basic physiological model and a knowledge-based system as two sources of knowledge (the physiological situation and medical guidelines) seems reasonable. Further development of commercial systems for automated control of mechanical ventilation may benefit from the experience we made with the described prototype. The practicability, efficacy, and usability of the system have to be examined in randomized controlled trials using different automated controlled mechanical ventilation systems.
References
Dreyfuss D, Saumon G. Ventilator-induced lung injury: lessons from experimental studies. Am J Respir Crit Care Med. 1998;157:294–323.
Levine S, Nguyen T, Taylor N, Friscia ME, Budak MT, Rothenberg P, Zhu J, Sachdeva R, Sonnad S, Kaiser LR, Rubinstein NA, Powers SK, Shrager JB. Rapid disuse atrophy of diaphragm fibers in mechanically ventilated humans. N Engl J Med. 2008;358:1327–35.
Ely EW, Baker AM, Dunagan DP, Burke HL, Smith AC, Kelly PT, Johnson MM, Browder RW, Bowton DL, Haponik EF. Effect on the duration of mechanical ventilation of identifying patients capable of breathing spontaneously. N Engl J Med. 1996;335:1864–9.
Grap MJ, Strickland D, Tormey L, Keane K, Lubin S, Emerson J, Winfield S, Dalby P, Townes R, Sessler CN. Collaborative practice: development, implementation, and evaluation of a weaning protocol for patients receiving mechanical ventilation. Am J Crit Care. 2003;12:454–60.
Henneman E, Dracup K, Ganz T, Molayeme O, Cooper C. Effect of a collaborative weaning plan on patient outcome in the critical care setting. Crit Care Med. 2001;29:297–303.
Horst HM, Mouro D, Hall-Jenssens RA, Pamukov N. Decrease in ventilation time with a standardized weaning process. Arch Surg. 1998;133:483–8.
Kollef MH, Shapiro SD, Silver P, St John RE, Prentice D, Sauer S, Ahrens TS, Shannon W, Baker-Clinkscale D. A randomized, controlled trial of protocol-directed versus physician-directed weaning from mechanical ventilation. Crit Care Med. 1997;25:567–74.
Marelich GP, Murin S, Battistella F, Inciardi J, Vierra T, Roby M. Protocol weaning of mechanical ventilation in medical and surgical patients by respiratory care practitioners and nurses: effect on weaning time and incidence of ventilator-associated pneumonia. Chest. 2000;118:459–67.
Saura P, Blanch L, Mestre J, Valles J, Artigas A, Fernandez R. Clinical consequences of the implementation of a weaning protocol. Intensive Care Med. 1996;22:1052–6.
Scheinhorn DJ, Chao DC, Stearn-Hassenpflug M, Wallace WA. Outcomes in post-ICU mechanical ventilation: a therapist-implemented weaning protocol. Chest. 2001;119:236–42.
Smyrnios NA, Connolly A, Wilson MM, Curley FJ, French CT, Heard SO, Irwin RS. Effects of a multifaceted, multidisciplinary, hospital-wide quality improvement program on weaning from mechanical ventilation. Crit Care Med. 2002;30:1224–30.
Tonnelier JM, Prat G, Le Gal G, Gut-Gobert C, Renault A, Boles JM, L’Her E. Impact of a nurses’ protocol-directed weaning procedure on outcomes in patients undergoing mechanical ventilation for longer than 48 h: a prospective cohort study with a matched historical control group. Crit Care. 2005;9:R83–9.
Vitacca M, Vianello A, Colombo D, Clini E, Porta R, Bianchi L, Arcaro G, Vitale G, Guffanti E, Lo Coco A, Ambrosino N. Comparison of two methods for weaning patients with chronic obstructive pulmonary disease requiring mechanical ventilation for more than 15 days. Am J Respir Crit Care Med. 2001;164:225–30.
Boles JM, Bion J, Connors A, Herridge M, Marsh B, Melot C, Pearl R, Silverman H, Stanchina M, Vieillard-Baron A, Welte T. Weaning from mechanical ventilation. Eur Respir J. 2007;29:1033–56.
MacIntyre NR, Cook DJ, Ely EW Jr, Epstein SK, Fink JB, Heffner JE, Hess D, Hubmayer RD, Scheinhorn DJ. Evidence-based guidelines for weaning and discontinuing ventilatory support: a collective task force facilitated by the American College of Chest Physicians; the American Association for Respiratory Care; and the American College of Critical Care Medicine. Chest Suppl. 2001;6:375–95.
Ball J. Recently published papers: the Jekyll and Hyde of oxygen, neuromuscular blockade and good vibrations? Crit Care. 2007;11:108.
Gattinoni L, Caironi P, Cressoni M, Chiumello D, Ranieri VM, Quintel M, Russo S, Patroniti N, Cornejo R, Bugedo G. Lung recruitment in patients with the acute respiratory distress syndrome. N Engl J Med. 2006;354:1775–86.
Huang CC, Shih MJ, Tsai YH, Chang YC, Tsao TC, Hsu KH. Effects of inverse ratio ventilation versus positive end-expiratory pressure on gas exchange and gastric intramucosal PCO(2) and pH under constant mean airway pressure in acute respiratory distress syndrome. Anesthesiology. 2001;95:1182–8.
Putensen C, Muders T, Varelmann D, Wrigge H. The impact of spontaneous breathing during mechanical ventilation. Curr Opin Crit Care. 2006;12:13–8.
Whitehead T, Slutsky AS. The pulmonary physician in critical care × 7: ventilator induced lung injury. Thorax. 2002;57:635–42.
Ramsay MA, Savege TM, Simpson BR, Goodwin R. Controlled sedation with alphaxalone-alphadolone. BMJ. 1974;2:656–9.
Slutsky AS. Consensus conference on mechanical ventilation—January 28–30, 1993 at Northbrook, Illinois, USA. Part I. European society of intensive care medicine, the ACCP and the SCCM. Intensive Care Med. 1994;20:64–79.
Brunner JX. Principles and history of closed-loop controlled ventilation. Respir Care Clin N Am. 2001;7:341–62.
Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. The acute respiratory distress syndrome network. N Engl J Med. 2000;342:1301–8.
Gillbe CE, Salt JC, Branthwaite MA. Pulmonary function after prolonged mechanical ventilation with high concentrations of oxygen. Thorax. 1980;35:907–13.
Tehrani FT. Automatic control of mechanical ventilation. Part 1: theory and history of the technology. J Clin Monit Comput. 2008;22:409–15.
Briel M, Meade M, Mercat A, Brower RG, Talmor D, Walter SD, Slutsky AS, Pullenayegum E, Zhou Q, Cook D, Brochard L, Richard JC, Lamontagne F, Bhatnagar N, Stewart TE, Guyatt G. Higher versus lower positive end-expiratory pressure in patients with acute lung injury and acute respiratory distress syndrome: systematic review and meta-analysis. JAMA, J Am Med Assoc. 2010;303:865–73.
Brower RG, Lanken PN, MacIntyre N, Matthay MA, Morris A, Ancukiewicz M, Schoenfeld D, Thompson BT. Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med. 2004;351:327–36.
Caironi P, Cressoni M, Chiumello D, Ranieri M, Quintel M, Russo SG, Cornejo R, Bugedo G, Carlesso E, Russo R, Caspani L, Gattinoni L. Lung opening and closing during ventilation of acute respiratory distress syndrome. Am J Respir Crit Care Med. 2010;181:578–86.
Amato MB, Barbas CS, Medeiros DM, Magaldi RB, Schettino GP, Lorenzi-Filho G, Kairalla RA, Deheinzelin D, Munoz C, Oliveira R, Takagaki TY, Carvalho CR. Effect of a protective-ventilation strategy on mortality in the acute respiratory distress syndrome. N Engl J Med. 1998;338:347–54.
Villar J. Low versus high positive end-expiratory pressure in the ventilatory management of acute lung injury. Minerva Anestesiol. 2006;72:357–62.
Papadakos PJ, Halloran W, Hessney JI, Lund N, Feliciano DV. The use of pressure-controlled inverse ratio ventilation in the surgical intensive care unit. J Trauma. 1991;31:1211–4.
Kazmaier S, Rathgeber J, Buhre W, Buscher H, Busch T, Mensching K, Sonntag H. Comparison of ventilatory and haemodynamic effects of BIPAP and S-IMV/PSV for postoperative short-term ventilation in patients after coronary artery bypass grafting. Eur J Anaesthesiol. 2000;17:601–10.
Rathgeber J, Schorn B, Falk V, Kazmaier S, Spiegel T, Burchardi H. The influence of controlled mandatory ventilation (CMV), intermittent mandatory ventilation (IMV) and biphasic intermittent positive airway pressure (BIPAP) on duration of intubation and consumption of analgesics and sedatives. A prospective analysis in 596 patients following adult cardiac surgery. Eur J Anaesthesiol. 1997;14:576–82.
Putensen C, Mutz NJ, Putensen-Himmer G, Zinserling J. Spontaneous breathing during ventilatory support improves ventilation-perfusion distributions in patients with acute respiratory distress syndrome. Am J Respir Crit Care Med. 1999;159:1241–8.
Hering R, Viehofer A, Zinserling J, Wrigge H, Kreyer S, Berg A, Minor T, Putensen C. Effects of spontaneous breathing during airway pressure release ventilation on intestinal blood flow in experimental lung injury. Anesthesiology. 2003;99:1137–44.
Putensen C, Zech S, Wrigge H, Zinserling J, Stuber F, Von Spiegel T, Mutz N. Long-term effects of spontaneous breathing during ventilatory support in patients with acute lung injury. Am J Respir Crit Care Med. 2001;164:43–9.
Fernando T, Cade J, Packer J. Automatic control of arterial carbon dioxide tension in mechanically ventilated patients. IEEE Trans Inf Technol Biomed. 2002;6:269–76.
Hewlett AM, Platt AS, Terry VG. Mandatory minute volume. A new concept in weaning from mechanical ventilation. Anaesthesia. 1977;32:163–9.
Jandre FC, Pino AV, Lacorte I, Neves JH, Giannella-Neto A. A closed-loop mechanical ventilation controller with explicit objective functions. IEEE Trans Biomed Eng. 2004;51:823–31.
Laubscher TP, Heinrichs W, Weiler N, Hartmann G, Brunner JX. An adaptive lung ventilation controller. IEEE Trans Biomed Eng. 1994;41:51–9.
Marsh WI, Smith WD. A flexible system for closed-loop ventilator development. J Med Syst. 1982;6:53–9.
Mitamura Y, Mikami T, Sugawara H, Yoshimoto C. An optimally controlled respirator. IEEE Trans Biomed Eng. 1971;18:330–7.
Smith DM, Mercer RR, Eldridge FL. Servo control of end-tidal CO2 in paralyzed animals. J Appl Physiol. 1978;45:133–6.
Strickland JH Jr, Hasson JH. A computer-controlled ventilator weaning system. Chest. 1991;100:1096–9.
Tehrani F, Rogers M, Lo T, Malinowski T, Afuwape S, Lum M, Grundl B, Terry M. A dual closed-loop control system for mechanical ventilation. J Clin Monit Comput. 2004;18:111–29.
Tehrani FT, Abbasi S. Evaluation of a computerized system for mechanical ventilation of infants. J Clin Monit Comput. 2009;23:93–104.
Tehrani FT, Roum JH. Flex: a new computerized system for mechanical ventilation. J Clin Monit Comput. 2008;22:121–30.
Kwok HF, Linkens DA, Mahfouf M, Mills GH. SIVA: a hybrid knowledge-and-model-based advisory system for intensive care ventilators. IEEE Trans Inf Technol Biomed. 2004;8:161–72.
Rees SE, Allerod C, Murley D, Zhao Y, Smith BW, Kjaergaard S, Thorgaard P, Andreassen S. Using physiological models and decision theory for selecting appropriate ventilator settings. J Clin Monit Comput. 2006;20:421–9.
Rutledge GW, Thomsen GE, Farr BR, Tovar MA, Polaschek JX, Beinlich IA, Sheiner LB, Fagan LM. The design and implementation of a ventilator-management advisor. Artif Intell Med. 1993;5:67–82.
Riley RL, Cournand A. Ideal alveolar air and the analysis of ventilation-perfusion relationships in the lungs. J Appl Physiol. 1949;1:825–47.
Dojat M, Brochard L, Lemaire F, Harf A. A knowledge-based system for assisted ventilation of patients in intensive care units. Int J Clin Monit Comput. 1992;9:239–50.
Dojat M, Pachet F, Guessoum Z, Touchard D, Harf A, Brochard L. NeoGanesh: a working system for the automated control of assisted ventilation in ICUs. Artif Intell Med. 1997;11:97–117.
Dojat M, Harf A, Touchard D, Lemaire F, Brochard L. Clinical evaluation of a computer-controlled pressure support mode. Am J Respir Crit Care Med. 2000;161:1161–6.
Dojat M, Harf A, Touchard D, Laforest M, Lemaire F, Brochard L. Evaluation of a knowledge-based system providing ventilatory management and decision for extubation. Am J Respir Crit Care Med. 1996;153:997–1004.
Lellouche F, Mancebo J, Jolliet P, Roeseler J, Schortgen F, Dojat M, Cabello B, Bouadma L, Rodriguez P, Maggiore S, Reynaert M, Mersmann S, Brochard L. A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med. 2006;174:894–900.
Rose L, Presneill JJ, Johnston L, Cade JF. A randomised, controlled trial of conventional versus automated weaning from mechanical ventilation using SmartCare/PS. Intensive Care Med. 2008;34:1788–95.
Schadler D, Engel C, Elke G, Pulletz S, Haake N, Frerichs I, Zick G, Scholz J, Weiler N. Automatic control of pressure support for ventilator weaning in surgical intensive care patients. Am J Respir Crit Care Med. 2012;185:637–44.
Brunner JX, Iotti GA. Adaptive support ventilation (ASV). Minerva Anestesiol. 2002;68:365–8.
Campbell RS, Branson RD, Johannigman JA. Adaptive support ventilation. Respir Care Clin N Am. 2001;7:425–40, ix.
Chatburn RL, Mireles-Cabodevila E. Closed-loop control of mechanical ventilation: description and classification of targeting schemes. Respir Care. 2011;56:85–102.
Tehrani FT. Method and apparatus for controlling an artificial respirator. US Patent No. 4,986,268, issued Jan 22, 1991.
Tehrani FT. Automatic control of an artificial respirator. Proc IEEE EMBS Conf. 1993;1991(13):1738–9.
Belliato M, Palo A, Pasero D, Iotti GA, Mojoli F, Braschi A. Evaluation of adaptive support ventilation in paralysed patients and in a physical lung model. Int J Artif Organs. 2004;27:709–16.
Tassaux D, Dalmas E, Gratadour P, Jolliet P. Patient-ventilator interactions during partial ventilatory support: a preliminary study comparing the effects of adaptive support ventilation with synchronized intermittent mandatory ventilation plus inspiratory pressure support. Crit Care Med. 2002;30:801–7.
Dongelmans DA, Veelo DP, Paulus F, de Mol BA, Korevaar JC, Kudoga A, Middelhoek P, Binnekade JM, Schultz MJ. Weaning automation with adaptive support ventilation: a randomized controlled trial in cardiothoracic surgery patients. Anesth Analg. 2009;108:565–71.
Gruber PC, Gomersall CD, Leung P, Joynt GM, Ng SK, Ho KM, Underwood MJ. Randomized controlled trial comparing adaptive-support ventilation with pressure-regulated volume-controlled ventilation with automode in weaning patients after cardiac surgery. Anesthesiology. 2008;109:81–7.
Petter AH, Chiolero RL, Cassina T, Chassot PG, Muller XM, Revelly JP. Automatic “respirator/weaning” with adaptive support ventilation: the effect on duration of endotracheal intubation and patient management. Anesth Analg. 2003;97:1743–50.
Sulzer CF, Chiolero R, Chassot PG, Mueller XM, Revelly JP. Adaptive support ventilation for fast tracheal extubation after cardiac surgery: a randomized controlled study. Anesthesiology. 2001;95:1339–45.
Arnal JM, Wysocki M, Novotni D, Demory D, Lopez R, Donati S, Granier I, Corno G, Durand-Gasselin J. Safety and efficacy of a fully closed-loop control ventilation [IntelliVent-ASV(R)] in sedated ICU patients with acute respiratory failure: a prospective randomized crossover study. Intensive Care Med. 2012;38:781–7.
Lumb A. Nunn’s applied respiratory physiology. Oxford: Butterworth-Heinemann Ltd.; 2005.
Conflict of interest
The development of the system and the presented selected case study as well as the conduction of another randomized controlled trial were kindly supported by restricted research grants from Drägerwerk AG & Co. KGaA, Lübeck, Germany. DS and NW received lecture fees from Dräger Medical Deutschland GmbH. SM is an employee of Dräger Medical GmbH, Lübeck, Germany.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Schädler, D., Mersmann, S., Frerichs, I. et al. A knowledge- and model-based system for automated weaning from mechanical ventilation: technical description and first clinical application. J Clin Monit Comput 28, 487–498 (2014). https://doi.org/10.1007/s10877-013-9489-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10877-013-9489-7