Abstract
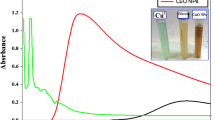
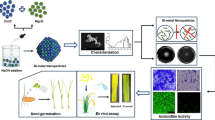
The novelty of this work is to estimate the antibacterial and antibiofilm capabilities of the mono-dispersed copper oxide nanoparticles (CuO NPs)-streptomycin nano-drug which synthesized by a cost-effective and eco-friendly gamma irradiation method. The incorporated CuO NPs-streptomycin was fully defined by UV–Vis., XRD, FTIR, HRTEM, DLS, HRSEM and EDX elemental analysis. In vitro antibacterial and antibiofilm activities of CuO NPs-streptomycin were examined towards pathogenic bacteria-causing brown, ring, soft rot and black leg diseases in potato plant. The proposed reaction mechanism regarding the synergistic potential between CuO NPs and streptomycin was estimated. The incorporated CuO NPs-streptomycin displayed an absorption peak at 585.0 nm specific for the Surface Plasmon Resonance. Results achieved from HRTEM, HRSEM and XRD verified the mono-dispersed crystalline character of the fabricated CuO NPs-streptomycin with a common particle size of 20.20 nm. CuO NPs-streptomycin exhibited an encouraging antibacterial activity against Clavibacter michiganensis subsp. sepedonicus (35.50 mm ZOI). Additionally, CuO NPs-streptomycin displayed enhanced biofilm inhibition percentage of about 90.99%, 84.23%, and 83.42% toward C. michiganensis subsp. sepedonicus, Ralstonia solanacearum, and Dickeya solani, respectively. Consequently, according to the prominent characteristics, this research could provide insights for determining dangerous agricultural challenges, potato packaging and processing and new nano-drug formula for invading potato pathogenic bacteria through the cultivation.










Similar content being viewed by others
References
C. Beaulieu and F. Van Gijsegem (1990). J.0 Bacteriol.172, (3), 1569–1575.
A. Sletten and T. Rafoss Fire Blight in Norway—An Assessment of the Plant Health Risk for the Plant Disease Fire Blight in Norway (Bioforsk, Oslo, 2007).
M. Perombelon (2000). EPPO Bull.30, (3–4), 413–420.
E. Parkin (1956). Annu. Rev. Entomol.1, (1), 223–240.
F. Dadaşoğlu and R. Kotan (2017). J. Anim. Plant Sci.27, 647–654.
M. Pérombelon (1992). Neth. J. Plant Pathol.98, (2), 135–146.
I. Toth, et al. (2011). Plant Pathol.60, (3), 385–399.
J. M. van der Wolf and S. H. De Boer Bacterial pathogens of potato. Potato Biology and Biotechnology (Oxford, Elsevier, 2007), pp. 595–617.
M. C. Perombelon and A. Kelman (1980). Annu. Rev. Phytopathol.18, (1), 361–387.
M. Pérombelon (2002). Plant Pathol.51, (1), 1–12.
A. O. Charkowski The soft rot Erwinia. Plant-Associated Bacteria (Springer, Dordrecht, 2007), pp. 423–505.
F. Barras, F. van Gijsegem, and A. K. Chatterjee (1994). Annu. Rev. Phytopathol.32, (1), 201–234.
S. Reverchon and W. Nasser (2013). Environ. Microbiol. Rep.5, (5), 622–636.
S. Baghaee-Ravari, et al. (2011). Eur. J. Plant Pathol.129, (3), 413–425.
C. Picard, et al. (2019). BASE23, (1), 36–45.
I. Hadizadeh, et al. (2019). Plant Pathol.68, (2), 297–311.
N. F. Sommer, R. J. Fortlage, and D. C. Edwards (2002). Postharvest. Technol. Hortic. Crops3311, 197.
L. R. Jones, M. Miller, and E. Bailey Frost Necrosis of Potato Tubers, vol. 46 (Agricultural Experiment Station of the University of Wisconsin, Madison, 1919).
P. Ark (1946). Am. J. Potato Res.23, (5), 170–181.
V. Divya Rani and H. Sudini (2013). Int. J. Plant Anim. Environ. Sci.3, (4), 156–164.
R. Trias, et al. (2008). Int. Microbiol.11, (4), 231.
A. Makhlouf and R. Abdeen (2014). J. Biol. Agric. Healthc.4, (10), 31–44.
D. R. Fravel (1988). Annu. Rev. Phytopathol.26, (1), 75–91.
M. Salanoubat, et al. (2002). Nature415, (6871), 497.
E. Yabuuchi, et al. (1995). Microbiol. Immunol.39, (11), 897–904.
C. Allen, P. Prior, and A. Hayward Bacterial Wilt Disease and the Ralstonia solanacearum Species Complex (American Phytopathological Society (APS Press), St. Paul, 2005).
G. Granada and L. Sequeira (1983). Can. J. Microbiol.29, (4), 433–440.
S. Weller, et al. (2000). Appl. Environ. Microbiol.66, (7), 2853–2858.
A. Hayward (1991). Annu. Rev. Phytopathol.29, (1), 65–87.
E. Guchi (2015). World J. Agric. Res.3, (1), 34–42.
M. M. López, et al. (2003). Int. Microbiol.6, (4), 233–243.
D. Baer and N. C. Gudmestad (1993). Phytopathology83, (2), 157–163.
J. T. Seil and T. J. Webster (2012). Int. J. Nanomed.7, 2767.
A. El-Batal, et al. (2014). Br. J. Pharm. Res.4, (11), 1341.
M. A. Maksoud, et al. (2018). Mater. Sci. Eng. C92, 644–656.
A. F. El-Baz, et al. (2016). J. Basic Microbiol.56, (5), 531–540.
K. Pal, et al. (2019). Electron. Mater. Lett.15, (1), 84–101.
T. Thirugnanasambandan, et al. (2018). Nano-Struct Nano-Objects16, 224–233.
S. Sajjadifar, et al. (2019). Chem. Methodol.3, (2), 226–236.
K. Pal, et al. (2015). J. Mater. Chem. C3, (45), 11907–11917.
M. A. Elkodous, et al. (2019). J. Mater. Sci. Mater. Electron.30, (9), 8312–8328.
M. A. Elkodous, et al. (2018). Charact. Appl. Nanomater.. https://doi.org/10.24294/can.v1i2.585.
M. I. A. A. Maksoud, et al. (2019). J. Mater. Sci. Mater. Electron.30, (5), 4908–4919.
A. Khatua, et al. (2019). J. Clust. Sci.. https://doi.org/10.1007/s10876-019-01624-6.
A. I. El-Batal, et al. (2017). J. Clust. Sci.28, (3), 1083–1112.
M. Abd Elkodous, et al. (2019). Colloids Surf. B Biointerfaces180, 411–428.
M. Abd Elkodous, et al. (2019). J. Clust. Sci.30, (3), 531–540.
H. Barabadi, et al. (2019). J. Clust. Sci.. https://doi.org/10.1007/s10876-019-01554-3.
M. Saravanan, et al. (2018). Microb. Pathog.115, 57–63.
S. Laurent, et al. (2008). Chem. Rev.108, (6), 2064–2110.
C. Xu and X. Qu (2014). NPG Asia Mater.6, (3), e90.
D. Ling and T. Hyeon (2013). Small9, (9–10), 1450–1466.
J. W. Rasmussen, et al. (2010). Expert Opin. Drug Deliv.7, (9), 1063–1077.
V. Ravishankar Rai, and A. Jamuna Bai Nanoparticles and their potential application as antimicrobials. A. Méndez-Vilas (ed.), Science Against Microbial Pathogens: Communicating Current Research and Technological Advances (Formatex, Mysore, 2011).
M. Ghorab, et al. (2016). Br. Biotechnol. J.16, (1), 1–25.
K. Pal, et al. (2015). Appl. Surf. Sci.357, 1499–1510.
D. V. Ponnuvelu, et al. (2016). Mater. Res. Express3, (10), 105005.
G. S. El-Sayyad, et al. (2019). J. Clust. Sci.. https://doi.org/10.1007/s10876-019-01629-1.
A. I. El-Batal, et al. (2019). J. Clust. Sci.. https://doi.org/10.1007/s10876-019-01619-3.
R. Emmanuel, et al. (2017). Microb. Pathog.113, 295–302.
M. Saravanan, et al. (2014). J. Bionanosci.8, (1), 21–27.
N. A. Dhas, C. P. Raj, and A. Gedanken (1998). Chem. Mater.10, (5), 1446–1452.
M.-S. Yeh, et al. (1999). J. Phys. Chem. B103, (33), 6851–6857.
M. Yang and J.-J. Zhu (2003). J. Cryst. Growth256, (1–2), 134–138.
P. Boomi, et al. (2019). J. Clust. Sci.30, (3), 715–726.
J. Cheon, J. Lee, and J. Kim (2012). Thin Solid Films520, (7), 2639–2643.
A. Pugazhendhi, et al. (2019). J. Photochem. Photobiol. B Biol.190, 86–97.
R. Balachandar, et al. (2019). J. Clust. Sci.30, 1–8.
K. Kanagamani, et al. (2019). J. Clust. Sci.30, (6), 1415–1424.
G. S. El-Sayyad, F. M. Mosallam, and A. I. El-Batal (2018). Adv. Powder Technol.29, (11), 2616–2625.
H. Barabadi, et al. (2019). J. Clust. Sci.. https://doi.org/10.1007/s10876-019-01668-8.
H. Barabadi, et al. (2014). Braz. J. Microbiol.45, (4), 1493–1501.
H. Barabadi, et al. (2017). Green Chem. Lett. Rev.10, (4), 285–314.
H. Barabadi, F. Kobarfard, and H. Vahidi (2018). Iran. J. Pharm. Res.17, (Special Issue 2), 87–97.
H. Barabadi, et al. (2019). J. Clust. Sci.30, (2), 259–279.
P. Kazakevich, et al. (2006). Appl. Surf. Sci.252, (13), 4373–4380.
G. Granata, et al. (2016). J. Nanoparticle Res.18, (5), 133.
C. Y. Ho, Y. H. Tsai, and F. M. Sui Thermal transport in the copper powders with nanometer and micrometer particles. Advanced Materials Research (Trans Tech Publ, Zurich, 2010).
J. B. Fathima, et al. (2018). J. Mol. Liq.260, 1–8.
S. N. Sinha, et al. (2015). Appl. Nanosci.5, (6), 703–709.
Z. Jiang, et al. (2019). Life Sci.220, 156–161.
N. Cioffi, et al. (2005). Anal. Bioanal. Chem.381, (3), 607–616.
R. G. Saratale, et al. (2018). J. Environ. Manag.223, 1086–1097.
S. Vasantharaj, et al. (2019). J. Photochem. Photobiol. B Biol.191, 143–149.
S. Sathiyavimal, et al. (2018). J. Photochem. Photobiol. B Biol.188, 126–134.
F. M. Mosallam, et al. (2018). Microb. Pathog.122, 108–116.
Z. Klencsár, et al. (2019). Mater. Chem. Phys.223, 122–132.
H. Nosrati, et al. (2018). Int. J. Biol. Macromol.108, 909–915.
M.-N. Yap Analysis of Erwinia Diversity and Cell Aggregation (The University of Wisconsin-Madison, Madison, 2006).
F. Casano, J. Wells, and T. Van der Zwet (1988). J. Phytopathol.121, (3), 267–274.
H. Jansing and K. Rudolph (1998). J. Plant Dis. Prot.105, 590–601.
Ahmed, M.E.E., Detection and effects of latent contamination of potato tubers by soft rot bacteria, and investigations on the effect of hydrogen peroxide on lipopolysaccharides of Erwinia carotovora in relation to acquired resistance against biocides. 2001, Citeseer.
H. A. Wahab and N. M. Balabel (2011). Egypt J. Phytopathol.39, (3), 154–168.
Doloman, A., Optimization of biogas production by use of a microbially enhanced inoculum. 2019.
J. Janse (1988). Eppo Bull.18, (3), 343–351.
G. Somodi, J. Jones, and J. Scott. Comparison of inoculation techniques for screening tomato genotypes for bacterial wilt resistance. in ACIAR Proceedings. (Australian Centre for International Agricultural Research, 1993).
J. Van der Wolf, et al., Epidemiology of Clavibacter michiganensis subsp. sepedonicus in relation to control of bacterial ring rot. 2005, PRI Bioscience.
R. Bryaskova, et al. (2011). J. Chem. Biol.4, (4), 185.
M. Balouiri, M. Sadiki, and S. K. Ibnsouda (2016). J. Pharm. Anal.6, (2), 71–79.
C. W. Wong, et al. (2019). J. Clust. Sci.. https://doi.org/10.1007/s10876-019-01651-3.
H. C. Diogo, et al. (2010). An. Bras. Dermatol.85, (3), 324–330.
A. I. El-Batal, et al. (2019). J. Clust. Sci.30, (4), 947–964.
A. I. El-Batal, F. M. Mosallam, and G. S. El-Sayyad (2018). J. Clust. Sci.29, 1–13.
M. Abd Elkodous, et al. (2019). Biol. Trace Elem. Res.. https://doi.org/10.1007/s12011-019-01894-1.
G. D. Christensen, et al. (1982). Infect. Immun.37, (1), 318–326.
M. A. Ansari, et al. (2014). Appl. Nanosci.4, (7), 859–868.
G. S. El-Sayyad, et al. (2019). Biol. Trace Elem. Res.. https://doi.org/10.1007/s12011-019-01842-z.
S. H. Abidi, et al. (2013). BMC Ophthalmol.13, (1), 57.
T. Mathur, et al. (2006). Indian J. Med. Microbiol.24, (1), 25.
K. Brownlee Probit Analysis: A Statistical Treatment of the Sigmoid Response Curve (Cambridge Univ. Press, New York, 1952).
N. Gudmestad and G. Secor Management of soft rot and ring rot. Potato Health Management (American Phytopathological Society, St. Paul, 1993), pp. 135–139.
M. Składanowski, et al. (2017). J. Clust. Sci.28, (1), 59–79.
S. Link and M. A. El-Sayed (2003). Ann. Rev. Phys. Chem.54, (1), 331–366.
G. Das, K.-H. Baek, and J. K. Patra (2019). PLoS ONE14, (6), e0217318.
N. Pauzi, N. M. Zain, and N. A. A. Yusof (2019). J. Environ. Chem. Eng.. https://doi.org/10.1016/j.jece.2019.103331.
N. Pauzi, N. M. Zain, and N. A. A. Yusof (2019). Bull. Chem. React. Eng. Catal.14, (1), 182–188.
L. Alrehaily, et al. (2013). Phys. Chem. Chem. Phys.15, (1), 98–107.
A. El-Batal, et al. (2016). J. Chem. Pharm. Res.8, (4), 934–951.
H. Barabadi, et al. (2019). Inorg. Nano-Metal Chem.49, (2), 33–43.
A. I. El-Batal, et al. (2019). J. Clust. Sci.30, (3), 687–705.
M. S. Attia, et al. (2019). J. Clust. Sci.30, (4), 919–935.
A. I. El-Batal, et al. (2016). Bioengineering3, (2), 14.
A. I. El-Batal, et al. (2017). J. Photochem. Photobiol. B Biol.173, 120–139.
A. El-Batal, et al. (2013). J. Chem. Pharm. Res.5, (8), 1–15.
A. I. El-Batal, et al. (2018). Microb. Pathog.118, 159–169.
A. Baraka, et al. (2017). Chem. Pap.71, (11), 2271–2281.
A. I. El-Batal, et al. (2016). Nanomater. Nanotechnol.6, 13.
A. El-Batal, et al. (2014). Br. J. Pharm. Res.4, (21), 2525.
A. Hanora, et al. (2016). J. Chem. Pharm. Res.8, (3), 405–423.
A. Ashour, et al. (2018). Particuology40, 141–151.
G. Govindasamy, et al. (2019). J. Mater. Sci. Mater. Electron.30, (17), 16463–16477.
P. K. Tiwari, et al. (2019). Ecotoxicol. Environ. Saf.176, 321–329.
H. Dong and G. M. Koenig (2019). CrystEngComm. https://doi.org/10.1039/C9CE00679F.
M. I. A. Abdel Maksoud, et al. (2019). J. Sol–Gel Sci. Technol.90, (3), 631–642.
P. Belavi, et al. (2012). Mater. Chem. Phys.132, (1), 138–144.
M. A. Maksoud, et al. (2019). J. Mater. Sci. Mater. Electron.30, 1–12.
K. Pal, M. A. Elkodous, and M. M. Mohan (2018). J. Mater. Sci. Mater. Electron.29, (12), 10301–10310.
M. Bashir and S. Haripriya (2016). Int. J. Biol. Macromol.93, 476–482.
E. R. Arakelova, et al. (2014). Int. J. Med. Heal. Pharm. Biomed. Eng.8, 33–38.
M. A. Maksoud, et al. (2019). Microb. Pathog.127, 144–158.
S. Pal, Y. K. Tak, and J. M. Song (2007). Appl. Environ. Microbiol.73, (6), 1712–1720.
A. Satyvaldiev, et al. Copper nanoparticles: synthesis and biological activity. IOP Conference Series: Materials Science and Engineering (IOP Publishing, Bristol, 2018).
V. Belava, et al. (2017). Nanoscale Res. Lett.12, (1), 250.
M. S. Rubina, et al. (2017). J. Nanostruct. Chem.7, (3), 249–258.
A. Pugazhendhi, et al. (2018). Microb. Pathog.122, 84–89.
C. Ashajyothi, et al. (2016). J. Nanostruct. Chem.6, (4), 329–341.
H.-J. Park, et al. (2013). Chemosphere92, (5), 524–528.
H. A. Ammar, G. H. Rabie, and E. Mohamed (2019). Bioprocess Biosyst. Eng.. https://doi.org/10.1007/s00449-019-02188-5.
E. Prokhorov, et al. (2019). Colloids Surf. B Biointerfaces180, 186–192.
P. K. Stoimenov, et al. (2002). Langmuir18, (17), 6679–6686.
M. F. Khan, et al. (2016). Sci. Rep.6, 27689.
A. Shinde, J. Ganu, and P. Naik (2012). J. Dental Allied Sci.1, (2), 63.
H. E. Alexander and G. Leidy (1947). J. Exp. Med.85, (4), 329–338.
W. Umbreit (1949). J. Biol. Chem.177, (2), 703–714.
H. Barabadi, et al. (2019). Medicina55, (8), 439.
K. Mortezaee, et al. (2019). Chem. Biol. Interact.312, 108814.
E. Assadian, et al. (2018). Biol. Trace Elem. Res.184, (2), 350–357.
R. Mani, et al. (2019). Environ. Sci. Pollut. Res.. https://doi.org/10.1007/s11356-019-06095-w.
S. Mahjouri, et al. (2018). Plant Cell Tissue Organ Cult.135, (2), 223–234.
K. Shahzad, et al. (2018). Environ. Sci. Pollut Res.25, (16), 15943–15953.
J.-K. Wan, et al. (2018). J. Appl. Phycol.30, (6), 3153–3165.
Acknowledgements
The authors would like to thank the Nanotechnology Research Unit (P.I. Prof. Dr. Ahmed I. El-Batal), Drug Microbiology Lab., Drug Radiation Research Department, NCRRT, Egypt, for financing and supporting this study under the project “Nutraceuticals and Functional Foods Production by using Nano/Biotechnological and Irradiation Processes”. Also, the authors would like to thank Prof. Mohamed Gobara (Military Technical College, Egyptian Armed Forces), and Zeiss microscope team in Cairo for their invaluable advice during this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
El-Batal, A.I., Balabel, N.M., Attia, M.S. et al. Antibacterial and Antibiofilm Potential of Mono-dispersed Stable Copper Oxide Nanoparticles-Streptomycin Nano-drug: Implications for Some Potato Plant Bacterial Pathogen Treatment. J Clust Sci 31, 1021–1040 (2020). https://doi.org/10.1007/s10876-019-01707-4
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-019-01707-4