Abstract
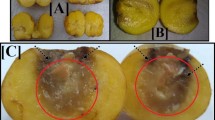
The purpose of this study is to investigate the ability of specific fungus to biosynthesize copper oxide nanoparticles (CuO NPs) by the aid of gamma rays and evaluate its performance as a unique antimicrobial agent in the agricultural fields. CuO NPs were synthesized by Penicillium chrysogenum filtrate utilizing copper sulfate at various gamma rays doses. The identification was performed by UV-Vis., FTIR, XRD, DLS, TEM, SEM, EDX and mapping images. Antimicrobial potential of CuO NPs against selected crop pathogenic microbes had been estimated. From the results, the preferred doses applied for CuO NPs synthesis was recorded at 50.0 kGy. The proposed reaction mechanism was studied. TEM image with DLS analysis confirmed the morphology of CuO NPs possesses a mean diameter at 9.70 nm. CuO NPs exhibited a maximum antifungal activity against Fusarium oxysporum (37.0 mm ZOI) followed by Alternaria solani (28.0 mm ZOI), and Aspergillus niger (26.5 mm ZOI). On the other hand, it was active as antibacterial agent against Ralstonia solanacearum (22.0 mm ZOI) and Erwinia amylovora (19.0 mm ZOI). Therefore, due to these outstanding properties, CuO NPs may be utilized as the significant antimicrobial agents in the agricultural area to restrain the plant pathogenic fungi and bacteria from proliferation.






Similar content being viewed by others
References
M. Abd Elkodous, et al. (2019). J. Clust. Sci.30, (3), 531–540.
K. Shameli, et al. (2012). Molecules17, (7), 8506–8517.
A. Pugazhendhi, et al. (2018). Int. J. Pharm.539, (1), 104–111.
M. Abd Elkodous, et al. (2019). Colloids Surf. B Biointerfaces180, 411–428.
M. I. A. Abdel Maksoud, et al. (2018). Mater. Sci. Eng. C92, 644–656.
M. A. Elkodous, et al. (2019). J. Mater. Sci. Mater. Electron.30, (9), 8312–8328.
A. A. Ponce and K. J. Klabunde (2005). J. Mol. Catal. A Chem.225, (1), 1–6.
M. Raffi, et al. (2010). Ann. Microbiol.60, (1), 75–80.
A. Pugazhendhi, et al. (2019). J. Photochem. Photobiol. B Biol.190, 86–97.
A. Ashour, et al. (2018). Particuology40, 141–151.
G. S. El-Sayyad, F. M. Mosallam, and A. I. El-Batal (2018). Adv. Powder Technol.29, (11), 2616–2625.
K. L. Kelly, et al. The Optical Properties of Metal Nanoparticles: The Influence of Size, Shape, and Dielectric Environment (ACS Publications, Washington, 2003).
D. Longano, et al. (2012) Synthesis and antimicrobial activity of copper nanomaterials. In: Cioffi N, Rai M (eds) Nano-Antimicrobials. Springer, Berlin, Heidelberg, pp 85–117. https://doi.org/10.1007/978-3-642-24428-5_3.
R. G. Saratale, et al. (2018). J. Environ. Manag.223, 1086–1097.
N. A. Dhas, C. P. Raj, and A. Gedanken (1998). Chem. Mater.10, (5), 1446–1452.
M.-S. Yeh, et al. (1999). J. Phys. Chem. B103, (33), 6851–6857.
M. Yang and J.-J. Zhu (2003). J. Cryst. Growth256, (1–2), 134–138.
J. Cheon, J. Lee, and J. Kim (2012). Thin Solid Films520, (7), 2639–2643.
P. Kazakevich, et al. (2006). Appl. Surf. Sci.252, (13), 4373–4380.
G. Granata, et al. (2016). J. Nanoparticle Res.18, (5), 133.
N. Asmathunisha and K. Kathiresan (2013). Colloids Surf. B Biointerfaces103, 283–287.
C.Y. Ho, Y.H. Tsai and F.M. Sui (2010). Adv. Mater. Res.126–128, 952–956. https://doi.org/10.4028/www.scientific.net/AMR.126-128.952.
J. B. Fathima, et al. (2018). J. Mol. Liquids260, 1–8.
S. N. Sinha, et al. (2015). Appl. Nanosci.5, (6), 703–709.
Z. Jiang, et al. (2019). Life Sci.220, 156–161.
N. Cioffi, et al. (2005). Anal. Bioanal. Chem.381, (3), 607–616.
S. Vasantharaj, et al. (2019). J. Photochem. Photobiol. B Biol.191, 143–149.
S. Sathiyavimal, et al. (2018). J. Photochem. Photobiol. B Biol.188, 126–134.
F. M. Mosallam, et al. (2018). Microb. Pathog.122, 108–116.
A. I. El-Batal, et al. (2019). J. Clust. Sci.30, (3), 687–705.
M. A. El-Bramawy (2006). Plant Prot. Sci. Prague42, (3), 99.
M. A. Khiyami, et al. (2014). Biotechnol. Biotechnol. Equip.28, (5), 775–785.
G. M. Mohamed, et al. (2010). J. Agric. Environ. Sci.9, (1), 1–23.
N. Hassan, et al. (2014). Mycobiology42, (4), 376–384.
M. A. Hassan and K. A. Abo-Elyousr (2013). Arch. Phytopathol. Plant Prot.46, (16), 1904–1918.
N. M. Hassanein, et al. (2010). Phytopathol. Mediterr.49, (2), 143–151.
A. El-Batal, et al. (2016). J. Chem. Pharm. Res.8, (4), 934–951.
A.-W. A. Ismail, et al. (2016). Br. Biotechnol. J.12, (3), 1.
V. Kumar, G. Singh, and A. Tyagi (2017). Int. J. Curr. Microbiol. Appl. Sci6, (5), 2343–2350.
M. A. Hegazi and G. A. El-Kot (2010). J. Agric. Sci.2, (4), 221.
P. Khalikar, G. Jagtap, and P. Sontakke (2011). Indian Phytopathol.64, (3), 286.
N. Akhtar, T. Anjum, and R. Jabeen (2013). Pak. Int. J. Agric. Biol.15, 1283–1288.
S. L. Leong, A. D. Hocking, and J. Pitt (2004). Aust. J. Grape Wine Res.10, (1), 83–88.
M. Abo-El-Dahab, et al. (1982). Phytopathol. Mediterr.1983, 168–170.
M. S. Attia, et al. (2019). J. Clust. Sci.30, (4), 919–935.
S. Kabeil, et al. (2008). World J. Agric. Sci.4, 803–810.
S. Kabeil, et al. (2008). Am. Eurasian J. Agric. Environ. Sci4, 44–54.
M. I. A. Abdel Maksoud, et al. (2019). J. Sol-Gel Sci. Technol.90, (3), 631–642.
A. Baraka, et al. (2017). Chem. Pap.71, (11), 2271–2281.
A. I. El-Batal, et al. (2019). J. Clust. Sci.30, (4), 947–964.
J. Ramyadevi, et al. (2012). Mater. Lett.71, 114–116.
M. I. A. A. Maksoud, et al. (2019). Microb. Pathog.127, 144–158.
A. I. El-Batal, F. M. Mosallam, and G. S. El-Sayyad (2018). J. Clust. Sci.29, (6), 1003–1015.
A. I. El-Batal, et al. (2017). J. Clust. Sci.28, (3), 1083–1112.
A. El-Batal, et al. (2014). Br. J. Pharm. Res.4, (11), 1341.
L. Alrehaily, et al. (2013). Phys. Chem. Chem. Phys.15, (1), 98–107.
A. I. El-Batal, et al. (2017). J. Photochem. Photobiol. B Biol.173, 120–139.
A. I. El-Batal, et al. (2018). Microb. Pathog.118, 159–169.
X. Zhu, et al. (2012). Langmuir28, (40), 14461–14469.
D. Das, et al. (2013). Colloids Surf. B Biointerfaces101, 430–433.
F. Rouxel, C. Alabouvette, and J. Louvet (1977). Ann. Phytopothol.9, (2), 183–192.
Zafar, M.B., et al. (2017) Fairness constraints: Mechanisms for fair classification. arXiv preprint arXiv:1507.05259.
J. Olchowik, et al. (2017). Forests8, (9), 310.
P. V. Viet, et al. (2016). J. Nanomater.2016, 6.
K. Bramhanwade, et al. (2016). Environ. Chem. Lett.14, (2), 229–235.
Y. He, et al. (2016). J. Nanobiotechnol.14, (1), 54.
M. Hildebrand, P. Aldridge, and K. Geider (2006). Mol. Genet. Genom.275, (3), 310–319.
K. Vrancken, et al. (2013). Microbiology159, (5), 823–832.
J. P. Ruparelia, et al. (2008). Acta Biomater.4, (3), 707–716.
A. Satyvaldiev, et al. (2018). IOP Conference Series: Materials Science and Engineering (IOP Publishing, 2018). https://doi.org/10.1088/1757-899X/302/1/012075.
Acknowledgements
The authors would like to thank the Nanotechnology Research Unit (P.I. Prof. Dr. Ahmed I. El-Batal), Drug Microbiology Lab., Drug Radiation Research Department, NCRRT, Egypt, for financing and supporting this study under the project “Nutraceuticals and Functional Foods Production by using Nano/ Biotechnological and Irradiation Processes”. Also, the authors would like to thank Prof. Mohamed gobara (Military Technical College, Cairo, Egypt), Dr. Mohamed S. Attia (Plant Pathology Lab., Botany and Microbiology Dep., Faculty of Science, Al-Azhar University, Cairo, Egypt) and Zeiss microscope team in Cairo, for their invaluable advice during this study.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human Participation and/or Animals
This article does not contain any studies with human and/or animals performed by any of the authors.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
El-Batal, A.I., El-Sayyad, G.S., Mosallam, F.M. et al. Penicillium chrysogenum-Mediated Mycogenic Synthesis of Copper Oxide Nanoparticles Using Gamma Rays for In Vitro Antimicrobial Activity Against Some Plant Pathogens. J Clust Sci 31, 79–90 (2020). https://doi.org/10.1007/s10876-019-01619-3
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10876-019-01619-3