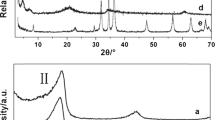
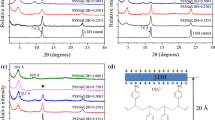
Abstract
Anionic surfactant intercalated layered double hydroxides (LDH) of high purity are easily prepared via direct coprecipitation and also by the ion exchange method provided that the precursor contains a monovalent anion, e.g., LDH–Cl or LDH–NO3. However, LDH–CO3 is an attractive starting material as it is commercially available in bulk form owing to large-scale applications as a PVC stabilizer and acid scavenger in polyolefins. Thus, intercalation of dodecyl sulfate and dodecylbenzenesulfonate into a commercial (LDH) with approximate composition [Mg0.654Al0.346(OH)2](CO3)0.173 · 0.5H2O] was explored. Direct ion exchange is difficult as the carbonate is held tenaciously. In the regeneration method it is removed by thermal treatment and the surfactant form obtained by reaction with the layered double hydroxide that forms in aqueous medium. Unfortunately the resulting products are impure, poorly crystallized and only partial intercalation is achieved. Better results were obtained using water-soluble organic acids, e.g., acetic, butyric, or hexanoic acid, to aid decarbonation of LDH–CO3. Intercalation proceeded at ambient temperatures with the precursor powder suspended in an aqueous dispersion of the anionic surfactant. The carboxylic acids are believed to assist intercalation by facilitating the elimination of the carbonate ions present in the anionic clay galleries.







Similar content being viewed by others
References
Brindley GW, Kikkawa S (1979) Am Mineral 64:836
Miyata S (1980) Clays Clay Miner 28:50. doi:https://doi.org/10.1346/CCMN.1980.0280107
Kopka H, Beneke K, Lagaly G (1988) J Colloid Interface Sci 123:427. doi:https://doi.org/10.1016/0021-9797(88)90263-9
Reichle WT (1986) Chemtech 16:58
Jones W, Chibwe M (1990) In: Mitchell IV (eds) Pillared layered structure: current trends and applications. Elsevier, London, p 67
Cavani F, Trifirò F, Vaccari A (1991) Catal Today 11:173. doi:https://doi.org/10.1016/0920-5861(91)80068-K
Miyata S, Kumura T (1973) Chem Lett 843. doi:https://doi.org/10.1246/cl.1973.843
Kanoh T, Shichi T, Takagi K (1999) Chem Lett 17. doi:https://doi.org/10.1246/cl.1999.117
Itoh T, Ohta N, Shichi T, Yui T, Takagi K (2003) Langmuir 19:9120. doi:https://doi.org/10.1021/la0302448
Xu ZP, Braterman PS (2003) J Mater Chem 13:268. doi:https://doi.org/10.1039/b207540g
Smyth JR, Bish DL (1988) Crystal structures and cation sites of rock forming minerals. Allen and Unwin, London, p 69
Bellotto M, Rebours B, Clause O, Lynch L (1996) J Phys Chem 100:8527. doi:https://doi.org/10.1021/jp960039j
Porter MR (1994) Handbook of surfactants, 2nd edn. Chapman and Hall
Tadros TF (2005) Applied surfactants principles and applications. Wiley-VCH Verlag GmbH and Co., Heppenheim, p 53
Crepaldi EL, Pavan PC, Tronto J, Cardoso LP, Valim JB (2002) J Colloid Interface Sci 248:429. doi:https://doi.org/10.1006/jcis.2002.8214
Harwell JH, Hoskins JC, Schechter RS, Wade WH (1985) Langmuir 1:251. doi:https://doi.org/10.1021/la00062a013
Bitting D, Harwell JH (1987) Langmuir 3:500
Chandler D (2005) Nature 437:640. doi:https://doi.org/10.1038/nature04162
Whitesides GM, Mathias JP, Seto CT (1991) Science 254:1312. doi:https://doi.org/10.1126/science.1962191
O’Hare D (1991) In: Bruce DW, O’Hare D (eds) Inorganic materials. Wiley, New York, p 167
Pavan PC, Crepaldi EL, Valim JB (2000) J Colloid Interface Sci 229:346. doi:https://doi.org/10.1006/jcis.2000.7031
Dèkány I, Haraszti T (1996) Colloid Surf A 123–124:391. doi:https://doi.org/10.1016/S0927-7757(96)03804-6
Pavan PC, Gomes GA, Valim JB (1998) Microporous Mesoporous Mater 21:659. doi:https://doi.org/10.1016/S1387-1811(98)00054-7
Pavan PC, Crepaldi EL, Gomes GA, Valim JB (1999) Colloid Surf A 154:399. doi:https://doi.org/10.1016/S0927-7757(98)00847-4
Ulibarri MA, Pavlovic I, Barriga C, Hermosín MC, Cornejo J (2001) Appl Clay Sci 18:17. doi:https://doi.org/10.1016/S0169-1317(00)00026-0
Anbarasan R, Lee WD, Im SS (2005) Bull Mater Sci 28:145. doi:https://doi.org/10.1007/BF02704234
Colvin VL, Goldstein AN, Alivisatos AP (1992) J Am Chem Soc 114:5221. doi:https://doi.org/10.1021/ja00039a038
Fendler JH, Meldrum FC (1995) Adv Mater 7:607. doi:https://doi.org/10.1002/adma.19950070703
Messersmith PB, Stupp SI (1995) Chem Mater 7:454. doi:https://doi.org/10.1021/cm00051a004
Dèkány I, Berger F, Imrik K, Lagaly G (1997) Colloid Polym Sci 275:681
Clearfield A, Kieke M, Kwan J, Colon JL, Wang RC (1991) J Incl Phenom Mol Recognit Chem 11:361. doi:https://doi.org/10.1007/BF01041414
Labajos FM, Rives V, Ulibarri MA (1992) J Mater Sci 27:1546. doi:https://doi.org/10.1007/BF00542916
Kloprogge JT, Hickey L, Frost RL (2002) J Mater Sci Lett 21:603. doi:https://doi.org/10.1023/A:1015655018529
Takagi K, Sichi T, Usami H, Sawaki Y (1993) J Am Chem Soc 115:4339. doi:https://doi.org/10.1021/ja00063a060
Yang K, Zhu L, Xing B (2007) Environ Pollut 145:571. doi:https://doi.org/10.1016/j.envpol.2006.04.024
Meyn M, Beneke K, Lagaly G (1990) Inorg Chem 29:5201
You Y, Zhao H, Vance GF (2002) Colloids Surf A Physicochem Eng Aspects 205:161. doi:https://doi.org/10.1016/S0927-7757(01)01137-2
Zhao H, Nagy KL (2004) J Colloid Interface Sci 274:613. doi:https://doi.org/10.1016/j.jcis.2004.03.055
Xu ZP, Braterman PS (2007) J Phys Chem 13:268
Pesic L, Salipurovic S, Markovic V, Vucelic D, Kagunya W, Jones W (1992) J Mater Chem 2:1069. doi:https://doi.org/10.1039/jm9920201069
Newman SP, Jones W (1998a) N J Chem 22:105. doi:https://doi.org/10.1039/a708319j
Drezdzon MA (1988) Inorg Chem 27:4628. doi:https://doi.org/10.1021/ic00298a024
Trujillano R, Holgado MJ, González JL, Rives V (2005) Solid State Sci 7:931. doi:https://doi.org/10.1016/j.solidstatesciences.2005.03.003
Hussein MZB, Zainal Z, Ming CY (2000) J Mater Sci Lett 19:879. doi:https://doi.org/10.1023/A:1006745716997
Crepaldi EL, Pavan PC, Valim JB (1999) Chem Commun (Camb) 155. doi:https://doi.org/10.1039/a808567f
Miyata S (1983) Clays Clay Miner 31:305. doi:https://doi.org/10.1346/CCMN.1983.0310409
Boehm H-P, Steinle J, Vieweger C (1977) Angew Chem 89:259. doi:https://doi.org/10.1002/ange.19770890416
Crepaldi EL, Pavan PC, Valim JB (2000) J Mater Chem 10:1337. doi:https://doi.org/10.1039/a909436i
Venugopal BR, Shivakumara G, Rajamathi M (2006) J Colloid Interface Sci 32:141
You Y, Zhao H, Vance GF (2002) J Mater Chem 12:907. doi:https://doi.org/10.1039/b106811c
Dimotakis ED, Pinnavaia TJ (1990) Inorg Chem 29:2393. doi:https://doi.org/10.1021/ic00338a001
Miyata S, Okada A (1977) Clays Clay Miner 25:14. doi:https://doi.org/10.1346/CCMN.1977.0250103
Sato T, Mikako T, Endo T, Shimada M (1987) J Chem Technol Biotechnol 39:275
Chibwe K, Jones W (1989) J Chem Soc Chem Commun 926. doi:https://doi.org/10.1039/c39890000926
Chen W, Qu B (2003) Chem Mater 15:3208. doi:https://doi.org/10.1021/cm030044h
Bouraada M, Lafjah M, Ouali MS, de Menorval LC (2008) J Hazard Mater 153:911. doi:https://doi.org/10.1016/j.jhazmat.2007.09.076
Costa FR, Leuteritz A, Wagenknecht U, Jehnichen D, Häusßler L, Heinrich G (2008) Appl Clay Sci 38:153. doi:https://doi.org/10.1016/j.clay.2007.03.006
Hansen HCB, Taylor RM (1991) Clay Miner 26:311. doi:https://doi.org/10.1180/claymin.1991.026.3.02
Carlino S, Hudson MJ (1994) J Mater Chem 4:99. doi:https://doi.org/10.1039/jm9940400099
Carlino S, Hudson MJ, Husain SW, Knowles JA (1996) Solid State Ionics 84:117. doi:https://doi.org/10.1016/S0167-2738(96)83014-1
Nhlapo N, Motumi T, Landman E, Verryn SMC, Focke WW (2008) J Mater Sci 43:1033. doi:https://doi.org/10.1007/s10853-007-2251-0
Anbarasan R, Lee WD, Im SS (2008) J Serb Chem Soc 73:321. doi:https://doi.org/10.2298/JSC0803321A
Vaccari A (1998) Catal Today 41:53. doi:https://doi.org/10.1016/S0920-5861(98)00038-8
Evans DG, Duan X (2006) Chem Commun (Camb) 485. doi:https://doi.org/10.1039/b510313b
Khan AI, O’Hare D (2002) J Mater Chem 12:3191. doi:https://doi.org/10.1039/b204076j
Leroux F, Besse J-P (2001) Chem Mater 13:3507. doi:https://doi.org/10.1021/cm0110268
Fischer H (2003) Mater Sci Eng C 23:763. doi:https://doi.org/10.1016/j.msec.2003.09.148
Costa FR, Abdel-Goad M, Wagenknecht U, Heinrich G (2005) Polymer (Guildf) 46:4447. doi:https://doi.org/10.1016/j.polymer.2005.02.027
Costa FR, Wagenknecht U, Jehnichen U, Abdel-Goad M, Heinrich G (2006) Polymer (Guildf) 47:1649. doi:https://doi.org/10.1016/j.polymer.2005.12.011
Wang B, Zhang H, Evans DG, Duan X (2005) J Mater Chem Phys 92:190. doi:https://doi.org/10.1016/j.matchemphys.2005.01.013
Choy J-H, Kwak S-Y, Park J-S, Jeong Y-J, Portier J (1999) J Am Chem Soc 121:1399. doi:https://doi.org/10.1021/ja981823f
Carlino S, Hudson MJ (1995) J Mater Chem 5:1433. doi:https://doi.org/10.1039/jm9950501433
Adachi-Pagano M, Forano C, Besse J-P (2000) Chem Commun (Camb) 91. doi:https://doi.org/10.1039/a908251d
Reichle WT (1985) J Catal 94:547. doi:https://doi.org/10.1016/0021-9517(85)90219-2
Rey F, Fornés V, Rojo JM (1992) J Chem Soc Faraday Trans 88:2233. doi:https://doi.org/10.1039/ft9928802233
Frost RL, Ding Z, Martens WN, Kloprogge JT (2003) J Therm Anal Calorim 71:429. doi:https://doi.org/10.1023/A:1022835305846
Kandare E, Hossenlopp JM (2006) Inorg Chem 45:3766. doi:https://doi.org/10.1021/ic060071k
Coates J (2000) In: Meyer RA (ed) Encyclopedia of analytical chemistry. Wiley, Chichester, p 10815
Perez-Ramirez J, Mul G, Kapteijn F, Moulijn JA (2001) J Mater Sci 11:821
Kloprogge JT, Frost RL (1999) Phys Chem Chem Phys 1:1641. doi:https://doi.org/10.1039/a808496c
Kloprogge JT, Frost RL (2000) Appl Catal A 204:269. doi:https://doi.org/10.1016/S0926-860X(00)00697-9
Rajamathi JT, Ravishankar N, Rajamathi M (2005) Solid State Sci 7:195. doi:https://doi.org/10.1016/j.solidstatesciences.2004.10.034
Nakagaki M, Yokoyama S (1985) J Pharm Sci 74:1047
Bethell D, Fessey RE, Namwindwa E, Robetrts DW (2001) J Chem Soc Perkin Trans 2:1489. doi:https://doi.org/10.1039/b102957f
Angarska JK, Tachev KD, Kralchevsky PA, Mehreteab A, Broze G (1998) J Colloid Interface Sci 200:31. doi:https://doi.org/10.1006/jcis.1997.5334
Crepaldi EL, Tronto J, Cardoso LP, Valim JB (2002) Colloid Surf A 211:103. doi:https://doi.org/10.1016/S0927-7757(02)00233-9
Parker ML, Milestone NB, Newman RH (1995) Ind Eng Chem Res 34:1196. doi:https://doi.org/10.1021/ie00043a023
Bontchev RP, Liu S, Krumhansl JL, Voigt J, Nenoff TM (2003) Chem Mater 15:3669. doi:https://doi.org/10.1021/cm034231r
Bish DL (1980) Bull Mineral (Paris) 103:170
Iyi N, Matsumoto T, Kaneko Y, Kitamura K (2004) Chem Mater 16:2926. doi:https://doi.org/10.1021/cm049579g
Iyi N, Okamoto K, Kaneko Y, Matsumoto T (2005) Chem Lett 34:932. doi:https://doi.org/10.1246/cl.2005.932
Jobbágy M, Regazoni AE (2004) J Colloid Interface Sci 275:345. doi:https://doi.org/10.1016/j.jcis.2004.01.082
Hu G, O’Hare D (2005) J Am Chem Soc 127:17808. doi:https://doi.org/10.1021/ja0549392
Acknowledgements
The authors thank Mrs. C. Harnisch of the Leibniz-Institut für Polymerforschung, Dresden e.V. for the Py/GC/MS analysis. Financial support for this research, from the Institutional Research Development Programme (IRDP), the South African Cooperation Fund for Scientific and Technological Developments (NEPAD), the THRIP program of the Department of Trade and Industry and the National Research Foundation of South Africa, as well as Xyris Technology CC, is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Moyo, L., Nhlapo, N. & Focke, W.W. A critical assessment of the methods for intercalating anionic surfactants in layered double hydroxides. J Mater Sci 43, 6144–6158 (2008). https://doi.org/10.1007/s10853-008-2935-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10853-008-2935-0