Abstract
A major disadvantage of microalgal cultivation is limited biomass yields due to the autotrophic lifestyle of most microalgal species. Heterotrophic growth on a suitable carbon source and oxygen can overcome such limitations. The red microalga Galdieria sulphuraria strain 074G grows heterotrophically on glucose and a number of other carbon sources while constitutively producing photopigments, including the blue-colored phycocyanin, a natural food colorant. Galdieria sulphuraria strain 074G grew well on maltodextrins as well as on granular starch in combination with the enzyme cocktail Stargen002. The maltodextrin cultures produced 2 mg phycocyanin per gram substrate, being slightly more than on glucose. The phycocyanin extracted from maltodextrin-grown cultures was thermostable up to 55 °C. Maltodextrins can be a cheap alternative to glucose syrups for the production of phycocyanin as natural food colorant.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Microalgae attract much attentions as they produce various organic compounds such as lipids, starch, and pigments that can be used as a renewable resources in the production of biodiesel, food supplements, or coloring agents (Mulders et al. 2014; Raheem et al. 2015; Rasala and Mayfield 2015; Wang et al. 2015). Large-scale cultivation of microalgae is attractive as they have a rapid life cycle, can be grown in sea, brackish water, and even waste water and in particular, do not require arable land (Chisti 2007; Li et al. 2008; Larkum et al. 2012). A major disadvantage of microalgae is that most species grow strictly autotrophic using (sun)light as energy source and carbon dioxide to form new organic matter. Autotrophic growth results in limited biomass yields as the penetration of light is inversely proportional to the cell concentration (Eriksen 2008; Liang et al. 2009; Grobbelaar 2010). Heterotrophic growth does not suffer from such disadvantages and can give substantially higher growth rates and biomass yields, especially when specific cultivation strategies like fed-batch are applied (Morales-Sanchez et al. 2013). Only a limited number of microalgae are able to grow heterotrophically depending on the strain and culture conditions (Chen and Chen 2006), examples being Tetraselmis chuii (Lu et al. 2017), Chlamydomonas reindhardtii (Zhang et al. 2019), Nitzschia laevis (Wen and Chen 2002), and Neochloris oleoabundans (Morales-Sanchez et al. 2013).
In most microalgae that grow heterotrophically, the production of photopigments is suppressed (Yamane et al. 2001; Bhatnagar et al. 2011). One of the few exceptions to this is Galdieria sulphuraria strain 074G, which constitutively produces photopigments when growing autotrophically in the light as well as heterotrophically in the dark (Gross and Schnarrenberger 1995). Besides chlorophyll, G. sulphuraria produces the blue-colored phycocyanin, a photopigment of the phycobilisomes, a light-harvesting complex found in Cyanobacteria, Cryptophyceae, and Rhodophyceae (Sekar and Chandramohan 2008). Currently the phycocyanin extracted from autotrophically grown Spirulina platensis is commercially available as a food colorant (Kamble et al. 2013). The considerably lower amount of phycocyanin per cell in heterotrophic cultures of G. sulphuraria 074G is compensated by much higher biomass yields (Graverholt and Eriksen 2007). An additional advantage offered by G. sulphuraria 074G is that it produces phycocyanin at a much higher rate than S. platensis (Pushparaj et al. 1997; Jiménez et al. 2003). The higher production rate and the higher yield make heterotrophic, high cell density cultivation of G. sulphuraria 074G attractive as an industrial-scale production system for phycocyanin.
So far, G. sulphuraria 074G has been grown only on low molecular weight carbon sources glucose, glycerol, or sucrose (Sloth et al. 2006; Schmidt et al. 2005). Glucose syrups used for high cell density fermentations are derived from starch by cooking the starch followed by a multistep enzymatic conversion (van der Maarel et al. 2002). Starch is a mixture of the glucose polymers amylose and amylopectin (van der Maarel et al. 2002). The granular starch is first gelatinized by jet-cooking to destroy the granular structure and bring the amylose and amylopectin in solution. Subsequently, the amylose and amylopectin are degraded by heat-stable α-amylase, liquefying the suspension, and an α-amylase-glucoamylase combination, resulting in complete saccharification. Shrestha and Weber (2007) showed that G. sulphuraria produces an extracellular glucoamylase, a glycoside hydrolase active at pH 2 and 80 °C converting amylose and amylopectin into glucose. In this paper, the growth of G. sulphuraria 074G on Paselli SA2, a potato starch maltodextrin produced by liquefaction of cooked potato starch, was tested, assuming that strain 074G also produces a glucoamylase that can convert the maltodextrin completely into glucose. Strain 074G grew very well on maltodextrins and a heat-stable phycocyanin could be extracted from the heterotrophically grown cells.
Material and methods
Strain and growth media
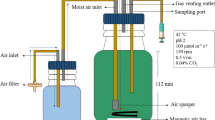
The red microalgae Galdieria sulphuraria strain 074G was obtained from AlgaeBiotech (Weesp, The Netherlands). A single colony was streaked onto Allen agar plate and grown for 2 weeks. One liter Allen medium (Allen 1959) contains 1.32 g (NH4)2SO4, 0.27 g KH2PO4, 0.25 g MgSO4.7H2O, 0.074 g CaCl2.2H2O, 11 mg FeCl3, 2.8 mg H3BO3, 1.8 mg MnCl2, 0.218 mg ZnSO4.7H2O, 0.05 mg CuSO4, 0.023 mg NH4VO3, and 0.023 mg NaMoO4.4H2O. The pH was adjusted to 2.0 with 4 M H2SO4, and the medium was sterilized by autoclaving at 121 °C for 20 min. Stock cultures were maintained by sub-cultivation in a mineral medium without organic carbon substrates under constant light (100 μmol photons m-2 s-1) at 150 rpm on a shaker incubator. Growth experiments were conducted in a 1 L working volume bioreactor with constant stirring at 150 rpm at 40 °C and complete darkness. Different carbon sources were used for heterotrophic growth: glucose (Sigma, USA), Paselli SA2, corn starch, sago starch, and potato starch (all from AVEBE, The Netherlands) with a final concentration of 10 g L−1. The enzyme Stargen 002 (Dupont Industrial Bioscience, The Netherlands) was added (0.5%, v/v) to the cultures supplemented with corn or potato starch. Different concentrations of glucose and Paselli SA2 (10 and 50 g L−1) were added for testing their effect on the biomass and pigment yield.
Paselli SA2 is a partially hydrolyzed potato starch with an average degree of polymerization of 50 (AVEBE, The Netherlands). Stargen 002 is a blend of Aspergillus kawachi glucoamylase and Trichoderma reesei α-amylase, activity minimum 570 glucoamylase unit (GAU) g−1, pH 3.3–4.5, and recommended minimum temperature is 48 °C (Genencor 2008). One GAU is the amount of enzyme that will release one gram of reducing sugars per hour from soluble starch substrate under specified condition (http://www.genencor.com).
Determination of algal growth parameters
The growth rate of cultures supplemented in corn or potato starch was monitored by cell counting. The number of cells was obtained using the improved Neubauer hemocytometer counting chamber and a light microscope. Around 200 μL of the culture was diluted, and the average cell count value was recorded as cells L−1. For the cultures supplemented with glucose or Paselli SA2, growth was measured by determining the optical density at 800 nm, at which pigment absorbance is negligible. The in vivo phycocyanin amount was determined by measuring the absorption at 618 nm and 652 nm. All experiments were performed in triplicate. Specific growth rates were calculated from growth curves as the slope of the linear regression of the natural log cell number versus time by Eq. 1 (Guillard and Ryther 1962):
where, OD0 refers to the OD value of early exponential (t0) and ODt is the OD value of late exponential (tt).
At the end of exponential phase, cells were harvested by centrifugation at 10,000×g for 5 min and subsequently dried algal biomass was obtained by freeze-drying.
Extraction and quality test of phycocyanin
Dried biomass was resuspended in 50 mM phosphate buffer pH 7.2 and disrupted with a high pressure homogenizer (Emulsiflex-B15, Avestin) for 5 cycles at 120 psi. The cell debris was removed by centrifugation at 24,000×g for 90 min at 4 °C and the blue-colored supernatant was collected in clean tubes. The phycocyanin content was measured spectrophotometrically. Phycocyanin and allophycocyanin have maximum absorption at 618 and 652, respectively. The concentration of phycocyanin in the solution was calculated using Eq. 2 (Bennet and Bogorad 1973):
The phycocyanin stability was evaluated as described previously with slight modification (Moon et al. 2014). 1 mL of phycocyanin solutions were incubated for 30 min at different temperatures (30, 40, 50, 60, 65, 70, and 80 °C). After incubation, the phycocyanin solutions were centrifuged to remove debris and the amount of phycocyanin in solution was measured spectrophotometrically. The remaining concentration of phycocyanin was calculated using Eq. 3 (Chaiklahan et al. 2012).
where C0 is initial concentration of phycocyanin, and C1 is phycocyanin concentration after treatment.
Results
Growth on maltodextrins and granular starches
As in the previous study by Schmidt et al (2005), G. sulphuraria was able to grow in various simple sugars as the carbon source, such as glucose, fructose, sucrose, and maltose in order to evaluate its pigmentation. In this study, effect of various complex sugar on biomass of strain 074G were investigated in a 1-L bioreactor on Allen medium under identical condition to the simple sugar substrate culture.
Growth of G. sulphuraria 074G on maltodextrin was compared with that with glucose at various concentrations. Figure 1 shows cell growth and substrate consumption of two comparative cultivations, using 10 g L−1 glucose and Paselli SA2 as growth substrate, respectively. Glucose cultures grew rapidly and within 7 days, reached their maximal cell density (OD800 = 15.3), while cells growth at Paselli SA2 showed extension of lag phase until the fourth day. As result, Paselli SA2 cultures had a slightly longer doubling time than glucose culture (20 h versus 17 h, respectively). In the media containing 10 g L−1 of Paselli SA2, it was observed that the substrate was not totally consumed, in opposition to the medium with 10 g L−1 D-glucose, where glucose gradually decreased over time and was almost completely depleted before the end of cultivation (14 days of growth) (Fig. 1).
As shown in Table 1, when supplemented with 50 g L−1 glucose, the maximum biomass of 4.9 ± 0.03 g L−1 with the growth rate (0.49 day−1) was achieved and was 1.04-fold higher than that obtained from 10 g L−1 glucose cultures. Interestingly, using a higher glucose concentration of 100 g L−1 did not provide a higher yield of biomass as the overall yield declined (data not shown). The yields (g g−1) (biomass per total sugar consumed; assuming 100% conversion of Paselli to glucose) of strain 074G increased sharply when the initial Paselli SA2 concentration was increased from 10 to 50 g L−1. However, no significant effect was observed in the yield of strain 074G when the glucose concentration was increased from 10 to 50 g L−1. The maximum biomass concentration obtained with Paselli SA2 at 50 g L−1 concentration as the carbon source was 6.5 g L−1.
Effect of various complex sugar on biomass of strain 074G were also investigated in a 1-L bioreactor on Allen medium containing granular starches together with Stargen 002 under identical condition to the simple sugar substrate culture. The influence of various starches on cell growth is presented in Fig. 2. Following transfer into growth medium, the lag phase of G. sulphuraria 074G on Allen medium containing corn-Stargen 002 was significantly shorter (2 days) than that of G. sulphuraria 074G on potato-Stargen 002 medium (3 days). Growth on a mixture of corn and Stargen 002 produced a growth rate (μ, 0.41 day−1) greater than on potato-Stargen 002 (μ, 0.38 day−1), but growth rate obtained from cultures grown on corn or potato only has very low values. The total glucose consumed (1.12 g L−1, conversion of starch to glucose) of corn-Stargen 002 culture was higher compared with cultures cultivated on other starches (data not shown). As a result, corn-Stargen 002 showed the fastest cell growth due to fast utilization of glucose. The specific growth rate, biomass production, and PC yield on various complex sugars were compared and summarized in Table 1.
Production and quality of phycocyanin
To demonstrate further the effect of increasing glucose concentration on phycocyanin production, the amount of glucose utilized and remaining was evaluated and measured. Sloth et al. (2006) demonstrated that heterotrophic batch culture of G. sulphuraria accumulates phycocyanin approximately 2–4 mg g−1 dry weight during exponential phase and higher when grown in fed-batch system (10–30 mg g−1 dry weight).
G. sulphuraria 074G grown on granular starch with or without Stargen 002 presented low growth rate compared with grown on complex sugar; therefore, phycoyanin production from these cultures was low. In cultures on granular starch, the highest phycocyanin production and efficiency of phycocyanin on substrate was potato-Stargen 002 culture, 1.1 ± 0.2 mg L−1 and 2.1 mg g−1, respectively. While cultures on glucose and Paselli SA2, the highest efficiency of phycocyanin on substrate were figured on 50 g L−1 glucose and 50 g L−1, 1.7 mg g−1 and 2.1 mg g−1, respectively, and phycocyanin production were 83 ± 5 mg L−1 and 104 ± 4 mg L−1. From these data, culture on Paselli SA2 gave highest phycocyanin production and it occurred on day 7.
Recently, phycocyanin is used as a coloring agent on food and beverages. For this application, critical temperature of phycocyanin is necessary to be determined. The effect of temperature on phycocyanin stability indicates that concentration of phycocyanin remained consistent until 50 °C, and decreased for 50% at 60 °C (Fig. 3). The decreasing of phycocyanin content in solution increased quickly after 55 °C.
Discussion
Growth on maltodextrins and granular starches
The productivity of several microalgal species when growing heterotrophically has been studied to achieve high amounts of biomass and high productivity of valuable bioproducts (Perez-Garcia et al. 2011). In this study, the phycocyanin production by G. sulphuraria strain 074G, a strain that maintains its photopigment production in the dark when growing on an organic carbon source, was investigated when maltodextrins or starch instead of glucose were supplied as the substrate. On the maltodextrin Paselli SA2, a slightly longer lag phase was observed compared with glucose. In addition, the Paselli SA2 culture grew slower reaching the stationary phase after 12 days, while the glucose culture reached the stationary phase after 6 days (Fig. 1). The highest production of microalgal biomass was achieved in 50 g L−1 Paselli SA2, whereas 10 g L−1 yielded the lowest amount of biomass. The result obtained here indicates a possible adaptation of algal to Paselli SA2 and it was feasible to use Paselli SA2 as carbon source to cultivate G. sulphuraria. As shown in Table 1, the specific growth rates for 10 and 50 g L−1 of initial glucose of strain 074G cultures were 0.72 and 0.49 day−1, respectively, showing that higher glucose concentrations inhibited growth. The lower specific growth was confirmed by a high amount of glucose (between 38.5 and 70.0 g L−1) present in the culture supernatant at the end of cultivation (data not shown).
The ability to grow on Paselli SA2 confirms the assumption that strain 074G produces one or more extracellular glycoside hydrolase(s) that converts the Paselli SA2 maltodextrin into glucose, which is then taken up by the cells and converted into energy and organic matter. Shrestha and Weber (2007) showed by proteome analysis that 11 extracellular proteins are present in the culture medium of G. sulphuraria including a putative glucoamylase (E.C. 3.2.1.3; 1,4-α-D-glucan glucohydrolase). This glucoamylase is active at pH 2–2.5 and 80 °C and showed activity towards starch and maltodextrin. In the whole genome sequence of G. sulphuraria, a range of genes with high similarity to various glycoside hydrolases are present, including two glucoamylases (Gasu_25520 and Gasu_25530) and one β-amylase (E.C. 3.2.1.2; Gasu_04150), all three with a clear signal sequence, indicating that these enzymes are excreted.
Paselli SA2 is produced by treating cooked potato starch for a few minutes with a thermostable α-amylase. The advantage such maltodextrin offers is that cooked starch only has to be treated very briefly during passage from the jet-cooker to the fermentation tank containing G. sulphuraria instead of degrading the starch all the way to glucose. This way, less equipment is needed, thereby saving costs. The process would even be more straightforward if G. sulphuraria could be grown on uncooked, granular starch which is fed directly into the fermentation tank. Galdieria sulphuraria 074G did not grow on granular corn or potato starch (Fig. 2). However, when the raw starch degrading enzyme cocktail Stargen 002 was added together with the granular starch, growth was clearly observed (Fig. 2), with the growth rates only slightly lower than those on glucose or maltodextrins (Table 1).
Production and quality of phycocyanin
Glucose has been used as a substrate to grow G. sulphuraria 074G and produce phycocyanin (Sloth et al. 2006; Graverholt and Eriksen 2007; Sørensen et al. 2013). Sloth et al. (2006) demonstrated that heterotrophic G. sulphuraria 074G grown in batch accumulates approx. 2–4 mg phycocyanin g−1 dry weight during the exponential growth phase; much higher yields of phycocyanin were found when a fed-batch system with glucose as feed was used (10–30 mg g−1 dry weight). As was shown in this research, G. sulphuraria 074G is capable of growing on the maltodextrin Paselli SA2 and even granular potato or corn starch when the enzyme cocktail Stargen 002 was added (Table 1). On Paselli SA2, G. sulphuraria 074G produced equal amounts of phycocyanin (16 mg g−1 dry weight) as on glucose, being in the range of what Sloth et al. (2006) reported (Table 1). The volumetric productivity on Paselli SA2 (104 mg L−1) was slightly higher than that on glucose (83 mg L−1) (Table 1).
The phycocyanin productivity on granular starch is much lower than on maltodextrin or glucose (Table 1). However, the efficiency defined as the amount of phycocyanin per gram of substrate added on granular potato starch with Stargen 002 is similar to that of maltodextrin or glucose (Table 1). Although the number of cells on granular corn starch with Stargen 002 is comparable to potato starch with Stargen 002, much less phycocyanin could be extracted from the corn starch culture. The phycocyanin extracted from maltodextrin-grown cells did not differ from that of glucose-grown cells; the overall absorption spectrum from 300 to 800 nm showed no differences and a clear absorption maximum was found at 618 nm. These absorption spectra are very similar to those found for phycocyanin extracted from G. sulphuraria (autotrophic; Moon et al. 2014), S. platensis (Patel et al. 2005), Calothrix sp (Santiago-Santos Ma et al. 2004), and Anabaena sp. (Ramos et al. 2009).
As G. sulphuraria grows in acidic hot springs up to 56 °C (Toplin et al. 2008), it is likely that the phycocyanin is stable at higher temperatures. The phycocyanin extracted from cultures grown on glucose and on Paselli SA2 was exposed for 30 min at temperatures varying from 30 to 80 °C (Fig. 3). Up to 55 °C, both phycocyanin solutions remained clearly blue, with 90% remaining in soluble. At 60 °C, most of the phycocyanin precipitated and the solution turned almost colorless. This finding is consistent with Moon et al. (2014), since phycocyanin is a protein conjugate pigment, the heat-induced irreversible process of phycocyanin denaturation.
References
Allen MB (1959) Studies with Cyanidium caldarium, an anomalously pigmented chlorophyte. Arch Mikrobiol 32:270–277
Bennet A, Bogorad L (1973) Complementary chromatic adaptation in a filamentous blue green alga. J Cell Biol 58:419–435
Bhatnagar A, Chinnasamay S, Singh M, Das KC (2011) Renewable biomass production bt mixotrophic algae in the presence of various carbon source and wastewater. Appl Energy 88:3425–3431
Chailakhan R, Chirasuwan S, Singh M, Das KC (2012) Stability of phycyanin extracted from Spirulina sp.: Influence of temperature, pH and preservatives. Process Biochem 47:659–664
Chen GQ, Chen F (2006) Growing phototrophic cells without light. Biotechnol Lett 28:607–616
Chisti Y (2007) Biodiesel from microalgae. Biotechnol Adv 25:294–306
Eriksen NT (2008) Production of phycocyanin–a pigment with applications in biology, biotechnology, foods and medicine. Appl Microbiol Biotechnol 80:1–14
Genencor (2008) STARGENTM 002: granular starch hydrolyzing enzyme for ethanol production. Product information sheet-STARGEN 002 technical bulletin
Graverholt OS, Eriksen NT (2007) Heterotrophic high-cell-density fed-batch and continuous-flow cultures of Galdieria sulphuraria and production of phycocyanin. Appl Microbiol Biotechnol 77:69–75
Grobbelaar JU (2010) Microalgal biomass production: challenges and realities. Photosynth Res 106:135–144
Gross W, Schnarrenberger C (1995) Heterotrophic growth of two strains of the acido-thermophilic red alga Galdieria sulphuraria. Plant Cell Physiol 36:633–638
Guillard RR, Ryther JH (1962) Studies on marine planktonic diatom I Cytotella nana Hustedt and Detonula confervacea (Cleve) Gran. Can J Microbiol 8:229–239
Jiménez C, Cossío BR, Labella D, Niell FX (2003) The feasibility of industrial production of Spirulina (Arthospira) in southern Spain. Aquaculture 217:179–190
Kamble SP, Gaikar RB, Padalia RB, Shinde KD (2013) Extraction and purification of C-phycocyanin from dry Spirulina powder and evaluating its antioxidant, anticoagulation and prevention of DNA damage activity. J Appl Pharmaceut Sci 3:149–153
Larkum AWD, Ross IL, Kruse O, Hankamer B (2012) Selection, breeding and engineering of microalgae for bioenergy and biofuel production. Trends Biotechnol 30:198–205
Li Y, Horsman M, Wu N, Lan CQ, Dubois-Calero N (2008) Biocatalysts and bioreactor design. Biotechnol Prog 24:815–820
Liang Y, Sarkany N, Cui Y (2009) Biomass and lipid productivities of Chlorella vulgaris under autotrophic, heterotrophic and mixotrophic growth conditions. Biotechnol Lett 31:1043–1049
Lu L, Wang J, Yang G, Zhu B, Pan K (2017) Heterotrophic growth and nutrient productivities of Tetraselmis chuii using glucose as a carbon source under different C/N ratios. J Appl Phycol 29:15–21
Moon M, Mishra SK, Kim CW, Suh WI, Park MS, Yang J-W (2014) Isolation and characterization of thermostable phycocyanin from Galdieria sulphuraria. Kor J Chem Eng 31: 490–495
Morales-Sanchez D, Tinoco-Valencia R, Kyndt J, Martinez A (2013) Heterotrophic growth of Neochloris oleoabundans using glucose as a carbon source. Biotechnol Biofuels 6:1–12
Mulders KJM, Lamers PP, Martens DE, Wiffels RH (2014) Phototrophic pigment production with microalgae: biological constraints and opportunities. J Phycol 50:229–242
Patel A, Mishra S, Pawar R, Ghosh PK (2005) Purification and characterization of C-phycocyanin from cyanobacterial species of marine and freshwater habitat. Protein Expr Purif 40:248–255
Perez-Garcia O, Escalante FME, de-Bashan L, Bashan Y (2011) Heterotrophic cultures of microalgae: metabolism and potential products. Water Res 45:11–36
Pushparaj B, Pelosi E, Tredici MR, Pinzani E, Materassi R (1997) An integrated culture system for outdoor production of microalgae and cyanobacteria. J Appl Phycol 9:113–119
Raheem A, Wan Azlina WAKG, Taufiq Yap YH, Danquah MK, Harun R (2015) Thermochemical conversion of microalgal biomass for biofuel production. Renew Sust Energ Rev 49:990–999
Ramos A, Acién FG, Fernández-Sevilla JM, González CV, Bermejo R (2009) Large-scale isolation and purification of C-phycocyanin from the cyanobacteria Anabaena marina using expanded bed adsorption chromatography. J Chem Technol Biotechnol 85:783–792
Rasala BA, Mayfield SP (2015) Photosynthetic biomanufacturing in green algae; production of recombinant proteins for industrial, nutritional, and medical uses. Photosynth Res 123:227–239
Santiago-Santos Ma C, Ponce-Noyola T, Olvera-Ramírez R, Ortega-López J, Cañizares-Villanueva RO (2004) Extraction and purification of phycocyanin from Calothrix sp. Process Biochem 39:2047–2052
Schmidt RA, Wiebe MG, Eriksen NT (2005) Heterotrophic high cell-density fed-batch cultures of the phycocyanin-producing red alga Galdieria sulphuraria. Biotechnol Bioeng 90:77–84
Sekar S, Chandramohan M (2008) Phycobiliproteins as a commodity: trends in applied research, patents and commercialization. J Appl Phycol 20:113–136
Shrestha RP, Weber AP (2007) Acidothermophilic red microalga Galdieria sulphuraria: from genome to an extracellular glucoamylase active at extreme low pH and high temperature. PSA Abstract. J Phycol 43:23–24
Sloth JK, Wiebe MG, Eriksen NT (2006) Accumulation of phycocyanin in heterotrophic and mixotrophic cultures of the acidophilic red alga Galdieria sulphuraria. Enzyme Microb Technol 38:168–175
Sørensen L, Hantke A, Eriksen NT (2013) Purification of the photosynthetic pigment C-phycocyanin from heterotrophic Galdieria sulphuraria. J Sci Food Agric 93:2933–2938
Toplin JA, Norris, TB, Lehr CR, McDermott TR, Castenholz RW (2008) Biogeographic and phylogenetic diversity of thermoacidophilic Cyanidiales in Yellowstone National Park, Japan and New Zealand. Appl Environ Microbiol 74:2822–2833
Van der Maarel MJEC, van der Veen B, Uitdehaag JCM, Leemhuis H, Dijkhuizen L (2002) Properties and application of starch-converting enzyme of the α–amylase family. J Biotechnol 94:137–155
Wang HM, Chen CC, Huynh P, Chang J (2015) Exploring the potential of using algae in cosmetics. Bioresour Technol 184:355–362
Wen ZY, Chen F (2002) Continuous cultivation of the diatom Nitzschia laevis for eicosapentaenoic acid production: physiological study and process optimization. Biotechnol Prog 18:21–28
Yamane Y, Utsunomiya T, Watanabe M, Sasaki K (2001) Biomass production in mixotrophic culture of Euglena gracilis under acidic condition and its growth energetic. Biotechnol Lett 23:1223–1228
Zhang Z, Tan Y, Wang W, Bai W, Fan J, Huang J, Wan M, Li Y (2019) Efficient heterotrophic cultivation of Chlamydomonas reinhardtii. J Appl Phycol 31:1545–1554
Funding
DYR was supported by the Ubbo Emmius PhD Scholarship program of the University of Groningen.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Rahman, D.Y., Sarian, F.D. & van der Maarel, M.J.E.C. Biomass and phycocyanin content of heterotrophic Galdieria sulphuraria 074G under maltodextrin and granular starches–feeding conditions. J Appl Phycol 32, 51–57 (2020). https://doi.org/10.1007/s10811-019-01957-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10811-019-01957-9