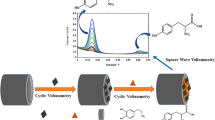
Abstract
In this study, an electrochemical sensor for the detection of herbicide diuron in natural water using a reduced graphene oxide–gold nanoparticle (rGO–AuNP)-modified screen-printed electrode (SPE) was developed. The proposed sensor was fabricated by the simple electrochemical co-reduction of graphene oxide and chloroauric acid using a cyclic voltammetry (CV) technique and applied to the direct electrochemical detection of diuron residue in water without using any mediator. A field emission scanning electron microscope image showed that the AuNPs were firmly attached and well distributed on the surface of the rGO nanosheets. The presence of rGO nanosheets was further proven by the Raman spectrum, which reveals that the D/G intensity ratio for rGO was smaller than that for GO. Moreover, the CV and linear sweep voltammetry results showed the effective accumulation of diuron on the rGO–AuNP/SPE surface and produced an irreversible reduction peak at around −0.5 V in a pH 5.5 phosphate buffer. Hence, it greatly enhanced the electrochemical reduction of diuron. Under optimized conditions, the cathodic peak current was proportional to the diuron concentration over a wide range of 0.5–30.0 µg mL−1, with a detection limit of 0.125 µg mL−1 (S/N = 3). The proposed diuron electrochemical sensor also exhibited a relative standard deviation of 4.25 % for a six replicate analysis of 10.0 µg mL−1 diuron, and the response of the electrode declined by 20 % after 30 days at ambient temperature. In addition, the sensor was successfully employed for the determination of diuron in a variety of water samples such as lake and seawater sample.
Graphical Abstract









Similar content being viewed by others
References
Hughes TP, Baird AH, Bellwood DR, Card M, Connolly SR, Folke C et al (2003) Climate change, human impacts, and the resilience of coral reefs. Science 301(5635):929–933
Watanabe T, Yuyama I, Yasumura S (2006) Toxicological effects of biocides on symbiotic and aposymbiotic juveniles of the hermatypic coral Acropora tenuis. J Exp Mar Biol Ecol 339(2):177–188
Stork PR, Bennett FR, Bell MJ (2008) The environmental fate of diuron under a conventional production regime in a sugarcane farm during the plant cane phase. Pest Manag Sci 64(9):954–963
Giacomazzi S, Cochet N (2004) Environmental impact of diuron transformation: a review. Chemosphere 56(11):1021–1032
Che D, Meacher RB, Rugh CL, Kim T, Heaton AC, Merkle SA (2006) Expression of organomercurial lyase in eastern cottonwood enhances organomercury resistance. Vitro Cell Dev Biol Plant 42(3):228–234
Hance R (1973) The effect of nutrients on the decomposition of the herbicides atrazine and linuron incubated with soil. Pest Sci 4(6):817–822
Fabricius KE (2005) Effects of terrestrial runoff on the ecology of corals and coral reefs: review and synthesis. Mar Pollut Bull 50(2):125–146
Katsumata H, Sada M, Nakaoka Y, Kaneco S, Suzuki T, Ohta K (2009) Photocatalytic degradation of diuron in aqueous solution by platinized TiO2. J Hazard Mater 171(1):1081–1087
Ong AS, Goh S (2002) Palm oil: a healthful and cost-effective dietary component. Food Nutri Bull 23(1):11–22
Sapozhnikova Y, Wirth E, Schiff K, Brown J, Fulton M (2007) Antifouling pesticides in the coastal waters of Southern California. Mar Pollut Bull 54(12):1972–1978
Feng J, Zheng Z, Luan J, Li K, Wang L, Feng J (2009) Gas–liquid hybrid discharge-induced degradation of diuron in aqueous solution. J Hazard Mater 164(2):838–846
Jones RJ, Kerswell AP (2003) Phytotoxicity of Photosystem II(PSII) herbicides to coral. Mar Ecol Prog Ser 261:149–159
Jones R (2005) The ecotoxicological effects of Photosystem II herbicides on corals. Mar Pollut Bull 51(5):495–506
Van Boven M, Laruelle L, Daenens P (1990) HPLC analysis of diuron and metabolites in blood and urine. J Anal Toxicol 14(4):231–234
Stan HJ (2000) Pesticide residue analysis in foodstuffs applying capillary gas chromatography with mass spectrometric detection: state-of-the-art use of modified DFG-multimethod S19 and automated data evaluation. J Chrom A 892(1):347–377
Draper WM (2001) Electrospray liquid chromatography quadrupole ion trap mass spectrometry determination of phenyl urea herbicides in water. J Agric Food Chem 49(6):2746–2755
Vessella J, Acobas F, Benanou D, Guinamant J (2001) Liquid chromatography coupled with time-of-flight mass spectrometery. The new way for environmental analysis. Spectr Anal 220:32–36
Rodríguez R, Picó Y, Font G, Mañes J (2001) Determination of urea-derived pesticides in fruits and vegetables by solid-phase preconcentration and capillary electrophoresis. Electrophoresis 22(10):2010–2016
Zhang M, Rassi ZE (2001) Capillary electrochromatography with polyacrylamide monolithic stationary phases having bonded dodecyl ligands and sulfonic acid groups: evaluation of column performance with alkyl phenyl ketones and neutral moderately polar pesticides. Electrophoresis 22(12):2593–2599
Berrada H, Font G, Moltó J (2001) Influence of the solvent on the gas chromatographic behaviour of urea herbicides. Chromatographia 54(3–4):253–262
Sanusi A, Millet M, Mirabel P, Wortham H (1999) Gas–particle partitioning of pesticides in atmospheric samples. Atmos Environ 33(29):4941–4951
Gerecke AC, Tixier C, Bartels T, Schwarzenbach RP, Müller SR (2001) Determination of phenylurea herbicides in natural waters at concentrations below 1 ng l−1 using solid-phase extraction, derivatization, and solid-phase microextraction–gas chromatography–mass spectrometry. J Chrom A 930(1):9–19
Wong A, Sotomayor MDPT (2014) Determination of carbofuran and diuron in FIA system using electrochemical sensor modified with organometallic complexes and graphene oxide. J Electroanal Chem 731:163–171
Védrine C, Fabiano S, Tran-Minh C (2003) Amperometric tyrosinase based biosensor using an electrogenerated polythiophene film as an entrapment support. Talanta 59(3):535–544
Sharma P, Bhalla V, Dravid V, Shekhawat G, Prasad ES, Suri CR (2012) Enhancing electrochemical detection on graphene oxide-CNT nanostructured electrodes using magneto-nanobioprobes. Sci Rep 877(2):1–7
Coelho-Moreira JDS, Bracht A, Souza ACDSD, Oliveira RF, Sá-Nakanishi ABD, Souza CGMD et al (2013) Degradation of diuron by Phanerochaete chrysosporium: role of ligninolytic enzymes and cytochrome P450. BioMed Res Int
Wittke K, Hajimiragha H, Dunemann L, Begerow J (2001) Determination of dichloroanilines in human urine by GC–MS, GC–MS–MS, and GC–ECD as markers of low-level pesticide exposure. J Chrom B 755(1):215–228
Novoselov KS, Geim AK, Morozov S, Jiang D, Zhang Y, Sa Dubonos et al (2004) Electric field effect in atomically thin carbon films. Sci 306(5696):666–669
Lee C, Wei X, Kysar JW, Hone J (2008) Measurement of the elastic properties and intrinsic strength of monolayer graphene. Science 321(5887):385–388
Balandin AA, Ghosh S, Bao W, Calizo I, Teweldebrhan D, Miao F et al (2008) Superior thermal conductivity of single-layer graphene. Nano Lett 8(3):902–907
Sun W, Lu Y, Wu Y, Zhang Y, Wang P, Chen Y et al (2014) Electrochemical sensor for transgenic maize MON810 sequence with electrostatic adsorption DNA on electrochemical reduced graphene modified electrode. Sens Act B 202:160–166
Lawal AT (2015) Synthesis and utilisation of graphene for fabrication of electrochemical sensors. Talanta 131:424–443
Choi BG, Park H, Park TJ, Yang MH, Kim JS, Jang S-Y et al (2010) Solution chemistry of self-assembled graphene nanohybrids for high-performance flexible biosensors. ACS Nano 4(5):2910–2918
Schniepp HC, Li J-L, McAllister MJ, Sai H, Herrera-Alonso M, Adamson DH et al (2006) Functionalized single graphene sheets derived from splitting graphite oxide. J Phys Chem B 110(17):8535–8539
Zhao H, Ji X, Wang B, Wang N, Li X, Ni R et al (2015) An ultra-sensitive acetylcholinesterase biosensor based on reduced graphene oxide-Au nanoparticles-β-cyclodextrin/Prussian blue-chitosan nanocomposites for organophosphorus pesticides detection. Biosens Bioelectron 65:23–30
Wu Y, Niu H, Wang K, Liu Q, Li H, Dong X et al (2013) Biosensor based on malic dehydrogenase immobilized in a CdS–graphene–chitosan nanocomposite for root-exuded malic acid determination. Sens Lett 11(2):436–441
Sun W, Cao L, Deng Y, Gong S, Shi F, Li G et al (2013) Direct electrochemistry with enhanced electrocatalytic activity of hemoglobin in hybrid modified electrodes composed of graphene and multi-walled carbon nanotubes. Anal Chim Acta 781:41–47
Song H, Ni Y, Kokot S (2013) A novel electrochemical biosensor based on the hemin–graphene nano-sheets and gold nano-particles hybrid film for the analysis of hydrogen peroxide. Anal Chim Acta 788:24–31
Ping J, Wang Y, Fan K, Wu J, Ying Y (2011) Direct electrochemical reduction of graphene oxide on ionic liquid doped screen-printed electrode and its electrochemical biosensing application. Biosens Bioelectron 28(1):204–209
Chen S, Murray RW (1999) Electrochemical quantized capacitance charging of surface ensembles of gold nanoparticles. J Phys Chem B 103(45):9996–10000
Li J, Yamada Y, Murakoshi K, Nakato Y (2001) Sustainable metal nano-contacts showing quantized conductance prepared at a gap of thin metal wires in solution. Chem Commun 21:2170–2171
Haruta M (1997) Size-and support-dependency in the catalysis of gold. Catal Today 36(1):153–166
Li J, Yang J, Yang Z, Li Y, Yu S, Xu Q et al (2012) Graphene–Au nanoparticles nanocomposite film for selective electrochemical determination of dopamine. Anal Methods 4(6):1725–1728
Yue-Rong W, Ping H, Liang Q-L, Guo-An L, Yi-Ming W (2008) Application of carbon nanotube modified electrode in bioelectroanalysis. Chin J Anal Chem 36(8):1011–1016
Won Y-H, Jang HS, Kim SM, Stach E, Ganesana M, Andreescu S et al (2009) Biomagnetic glasses: preparation, characterization, and biosensor applications. Langmuir 26(6):4320–4326
Huang N, Lim H, Chia C, Yarmo M, Muhamad M (2011) Simple room-temperature preparation of high-yield large-area graphene oxide. Int J Nanomed 6:3443
Madhu R, Dinesh B, Chen S-M, Saraswathi R, Mani V (2015) An electrochemical synthesis strategy for composite based ZnO microspheres–Au nanoparticles on reduced graphene oxide for the sensitive detection of hydrazine in water samples. RSC Adv 5(67):54379–54386
Bard AJ, Faulkner LR (1980) Electrochemical methods: fundamentals and applications. Wiley, New York
Yu H, Xu P, Lee D-W, Li X (2013) Porous-layered stack of functionalized AuNP–rGO (gold nanoparticles–reduced graphene oxide) nanosheets as a sensing material for the micro-gravimetric detection of chemical vapor. J Mater Chem A 1(14):4444–4450
Guo Y, Sun X, Liu Y, Wang W, Qiu H, Gao J (2012) One pot preparation of reduced graphene oxide (RGO) or Au (Ag) nanoparticle-RGO hybrids using chitosan as a reducing and stabilizing agent and their use in methanol electrooxidation. Carbon 50(7):2513–2523
Ferrari AC, Robertson J (2000) Interpretation of Raman spectra of disordered and amorphous carbon. Phys Rev B 61(20):14095
Stankovich S, Dikin DA, Piner RD, Kohlhaas KA, Kleinhammes A, Jia Y et al (2007) Synthesis of graphene-based nanosheets via chemical reduction of exfoliated graphite oxide. Carbon 45(7):1558–1565
Gan N, Yang X, Xie D, Wu Y, Wen W (2010) A disposable organophosphorus pesticides enzyme biosensor based on magnetic composite nano-particles modified screen printed carbon electrode. Sensors 10(1):625–638
Ping J, Wang Y, Ying Y, Wu J (2012) Application of electrochemically reduced graphene oxide on screen-printed ion-selective electrode. Anal Chem 84(7):3473–3479
Salinas-Torres D, Huerta F, Montilla F, Morallón E (2011) Study on electroactive and electrocatalytic surfaces of single walled carbon nanotube-modified electrodes. Electrochim Acta 56(5):2464–2470
Zheng Y, Wang A, Lin H, Fu L, Cai W (2015) A sensitive electrochemical sensor for direct phoxim detection based on an electrodeposited reduced graphene oxide–gold nanocomposite. RSC Adv 5(20):15425–15430
Chao M, Chen M (2014) Electrochemical determination of phoxim in food samples employing a graphene-modified glassy carbon electrode. Food Anal Methods 7(9):1729–1736
Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem and Interfacial Electrochem 101(1):19–28
Polcaro A, Palmas S, Dernini S (1993) Electrochemical reduction of carbonyl compounds at modified carbon felt electrodes. Electrochim Acta 38(2):199–203
Wang D-W, Gentle IR, Lu GQM (2010) Enhanced electrochemical sensitivity of PtRh electrodes coated with nitrogen-doped graphene. Electrochem Commun 12(10):1423–1427
Niesner R, Heintz A (2000) Diffusion coefficients of aromatics in aqueous solution. J Chem Eng Data 45(6):1121–1124
Johnson SL, Rumon K (1970) Ring-opening reaction of 1-(N, N-dimethylcarbamoyl) pyridinium chloride with hydroxide. A model for the alkaline diphosphopyridine nucleotide reaction. Biochemistry 9(4):847–857
Shi Q, Chen M, Diao G (2013) Electrocatalytical reduction of m-nitrophenol on reduced graphene oxide modified glassy carbon electrode. Electrochim Acta 114:693–699
Sharma P, Sablok K, Bhalla V, Suri CR (2011) A novel disposable electrochemical immunosensor for phenyl urea herbicide diuron. Biosens Bioelectron 26(10):4209–4212
Wong A, de Vasconcelos Lanza MR, Sotomayor MDPT (2013) Sensor for diuron quantitation based on the P450 biomimetic catalyst nickel (II) 1, 4, 8, 11, 15, 18, 22, 25-octabutoxy-29H, 31H-phthalocyanine. J Electroanal Chem 690:83–88
Bhalla V, Sharma P, Pandey SK, Suri CR (2012) Impedimetric label-free immunodetection of phenylurea class of herbicides. Sens Actuators B171:1231–1237
Sharma P, Bhalla V, Tuteja S, Kukkar M, Suri CR (2012) Rapid extraction and quantitative detection of the herbicide diuron in surface water by a hapten-functionalized carbon nanotubes based electrochemical analyzer. Analyst 137(10):2495–2502
Acknowledgments
The authors gratefully acknowledged the financial support of the Fundamental Research Grant Scheme (FRGS/1/2012/ST05/UPM/02/3) and High Impact Research Grant (UM.C/625/1/HIR/MOHE/SC/21) from the Ministry of Higher Education of Malaysia.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
10800_2016_950_MOESM1_ESM.tif
Supplementary material 1 (TIFF 490 kb). Figure S1. Raman spectra of GO, GO–AuNPs, and rGO–AuNPs after modification on SPE electrode
10800_2016_950_MOESM2_ESM.tif
Supplementary material 2 (TIFF 120 kb). Figure S2. Chronoamperometry curves obtained at rGO–AuNPs/SPE in presence of 10.0 mg ml−1 diuron recorded in PBS (0.01 mol L−1, pH 5.5). Inset: Cottrell plots obtained from the chronoamperometry curves
Rights and permissions
About this article
Cite this article
Shams, N., Lim, H.N., Hajian, R. et al. A promising electrochemical sensor based on Au nanoparticles decorated reduced graphene oxide for selective detection of herbicide diuron in natural waters. J Appl Electrochem 46, 655–666 (2016). https://doi.org/10.1007/s10800-016-0950-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10800-016-0950-4