Abstract
The emergence of reproductive isolation is key in maintaining within- and between-species diversity and one of the initial steps of speciation. In the Iberian Peninsula, the diverging populations of the Brachionus plicatilis rotifer create an ideal system to shed light on the mechanisms that give rise to the emergence of reproductive isolation. Herein, we quantify the degree of behavioural reproductive isolation in two groups of B. plicatilis populations, namely, neighbouring populations diverging by adaptation to the local environment and populations diverging in the absence of gene flow due to geographic distance. We conduct behavioural no-choice assays to test mating reproductive isolation between these populations. The analysis shows signatures of ongoing behavioural reproductive isolation in most of the population crosses, which is more pronounced in populations with a higher level of adaptive divergence, presumably under high migration rates. Overall, this study suggests that local adaptation is associated with mating behaviour resulting in reproductive isolation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reproductive isolation has strong implications for both genetic differentiation and evolutionary dynamics, affecting population structure, local adaptation, speciation and sister species coexistence (Coyne & Orr, 2004; Weber & Strauss, 2016). There is a wide range of intrinsic and extrinsic factors that could contribute to isolation. The causal loops that might occur among them make the dissection and testing of such a panoply complicated (Palumbi, 1994; Coyne & Orr, 2004).
One of the factors leading to reproductive isolation between populations may result from the difficulty of immigrants arriving, surviving and/or becoming sexually involved in the recipient population (Sexton et al., 2014; Turbek et al., 2018). If arrival rate is the prevalent factor, then a pattern of isolation by distance (IBD) is expected to emerge due to the combined, opposite effect of migration and genetic drift (Wright, 1943; Church & Taylor, 2002). On the other hand, if unmatched timings of dispersal/reproduction and survival are the prevalent factors, then a pattern of isolation by environment (IBE) is expected (Nosil et al., 2005; Wang & Bradburd, 2014). Local adaptation is likely to be involved in this second case (Nosil et al., 2002). IBD and IBE have been found in a broad range of organisms (IBD, e.g., Zeller et al., 2006; Mills et al., 2007; Campillo et al., 2011; Ventura et al., 2014; IBE, e.g., Shafer & Wolf, 2013; Martin et al., 2021) and may act together (Sexton et al., 2014; Weber et al., 2017). In both scenarios—IBD and IBE—low gene flow would promote behavioural reproductive isolation—i.e., based on mate preferences—due to genetic drift acting on genes that affect behaviour. Moreover, the genetic hitchhiking of behavioural genes linked to genes for local adaptation might play a role in the case of IBE (Hawthorne & Via, 2001; Wu, 2001, but see Parchman et al., 2013). In addition, if genetic divergence, either adaptive or nonadaptive, is associated with at least some degree of post-mating isolation, then selection for behavioural isolation would occur because it results in an optimized allocation of parental resources, i.e., avoiding the production of hybrid offspring. Thus, the degree of behavioural isolation is expected to increase with the geographic and environmental distance between populations and concomitantly with the genetic distance in neutral markers. Notably, regardless of how behavioural isolation arises, it is expected to reinforce genetic divergence in neutral markers, which illustrates the two-way causal links that might be at work.
A relatively high number of studies have focused on testing premating isolation between phylogenetically close species. This includes species studies in both vertebrates and invertebrates (Schröder & Walsh, 2010; Arthur & Dyer, 2015; Carranza et al., 2017; Luo et al., 2017; Cowles & Uy, 2019; Langton-Myers et al., 2019; Maltseva et al., 2021; Turbek et al., 2021) and has sometimes been associated with identifying cryptic speciation (Gomez & Serra, 1995; Schröder & Walsh, 2007; Juárez et al., 2015; Meguro et al., 2016; Castro Vargas et al., 2017; Ismail & Brooks, 2018). However, the evolution of premating isolation between populations within the same species has received much less attention (Jiggins et al., 2004; Sobel & Streisfeld, 2015; Cruz-Yepez et al., 2020). In this sense, microscopic aquatic invertebrates inhabiting spatially fragmented habitats such as lakes, ponds and lagoons offer an ideal model to study the effect of population isolation on reproductive isolation within species. Despite having the potential for high dispersal capability due to the small body size (< 1 mm length) (Bohonak & Jenkins, 2003; Louette & De Meester, 2005; Ventura et al., 2014), they show deep genetic divergence, which is a phenomenon known as the “dispersal-gene flow paradox” (De Meester et al., 2002). At the same time, living in a wide range of environmental conditions, local adaptation has been described in a variety of zooplankton species (Declerck et al., 2001; Declerck & Papakostas, 2016; Franch-Gras et al., 2017a).
Brachionus plicatilis Müller 1786 is a cyclical parthenogenetic rotifer that inhabits saline and brackish ponds. Its predominant type of reproduction is ameiotic parthenogenesis (Gilbert, 1963; Wallace et al., 2015; Serra et al., 2018). Its natural populations, which dwell in the water column temporarily within a year, are composed mainly of asexual (also called amictic) females. Sexual reproduction is initiated at high population densities (Stelzer & Snell, 2003) and starts with the production of sexual (mictic) daughters (Snell & Boyer, 1988). Sexual females, which produce meiotic eggs, have two mutually exclusive reproductive fates: to become male producers if not inseminated by a male while they are young or to become diapausing egg producers otherwise (Serra et al., 2018). Sexual and asexual females are morphologically identical, but they can be identified according to the eggs they carry.
Mating behaviour in rotifers is a three-step sequence: male–female encounter, circling and copulation. As rotifers do not secrete any sexual pheromones into the environment (Snell et al., 1995), male–female encounters occur randomly (Snell & Garman, 1986). After the encounter, the ciliated corona of the male contacts a female’s body (Snell & Morris, 1993; Snell et al., 1995), and the male displays a circling behaviour, i.e., swimming around the female while maintaining contact by his corona. After several circlings, the male locates either the female’s corona or the foot opening, and hypodermic insemination takes place (Gomez & Serra, 1995). The whole mating behaviour can take from tens of seconds to several minutes, and the three-step sequence can be interrupted at any time (Snell & Hawkinson, 1983; Snell & Hoff, 1987). A surface glycoprotein (mate recognition protein, MRP) present on the female’s body is involved in recognition of a mate (Snell & Morris, 1993; Snell et al., 1995; Gribble et al., 2011). Although mate choice is mostly driven by males, females may exhibit a reaction by varying their resistance to male circling (Snell et al., 2007). Female susceptibility to fertilization and male capacity for fertilization decline with age (Snell & Childress, 1987). Male rotifers tend to copulate more often with young females but show no preference for sexual over asexual females (Gomez & Serra, 1996).
In the Iberian Peninsula, populations of the cyclically parthenogenetic rotifer B. plicatilis create an ideal system for studying intrinsic factors resulting in some level of reproductive isolation. The species consists of highly divergent lineages (Gomez et al., 2000), reflecting strong founder effects with subsequent resource monopolization by the founder genotypes. Additionally, Iberian populations of B. plicatilis inhabit highly diversified habitats, from ephemeral—and highly environmentally unpredictable—to permanent ponds. They show local adaptation affecting their sexual reproduction patterns, adjusting them to variation in environmental predictability (i.e., high propensity to sex in populations with low environmental predictability Franch-Gras et al., 2017a, b). A recent study on the diversity of the gene mmr-b, which is involved in mate recognition, has shown that despite being under stabilizing selection, a correlation is found between gene diversification and differences in environmental factors when comparing B. plicatilis populations (Jezkova et al., 2022). Brachionus plicatilis belongs to a species complex, and the divergence of mmr-b among the species of the complex has also been documented (Gribble & Welch, 2012).
In this research, we tested the hypothesis that evolution of premating behavioural isolation occurs between populations of the species B. plicatilis. We hypothesized that the ecological divergence is correlated with that isolation. We used bidirectional no-choice mating tests to investigate within-species mating preferences in populations belonging to different phylogeographic groups or showing ecological divergence. The results were used to assess the consistency of different evolutionary hypotheses, and phylogeographical and ecological divergences were found to play a role in the establishment of behavioural reproductive isolation.
Material and methods
Study populations
We selected two groups of populations of B. plicatilis inhabiting shallow ponds in eastern Spain. One group was based on known phylogeographic structure, with each population belonging to a different clade, and the other was based on known differentiation in adaptation to local conditions (Table 1). For the group of phylogeographically structured populations (PS), we chose three populations, each with a dominant haplotype belonging to divergent mtDNA clades and showing a high degree of pairwise population differentiation (Gomez et al., 2002) (Table 2). The selected populations were Salada de Chiprana (CHI) from the northern clade, Torreblanca Norte (TON) from the coastal clade and Salobralejo (SAL) from the southern clade. For the group of locally adapted populations (LA), we chose three populations belonging to the same phylogeographic clade but differing in their propensity for sexual reproduction, which correlates with environmental unpredictability (Franch-Gras et al., 2017a, 2018). The selected populations were Hoya Yerba (HYB), Hoya Rasa (RAS) and Salobralejo (SAL), corresponding to low, medium and high predictability, respectively. The pairwise FST values of the populations and geographic distances between their localities are shown in Table 2.
Clone isolation and culture conditions
The sediment of the ponds was sampled in 2013. Diapausing eggs were extracted from the sediment in 2017 using a sugar flotation technique (i.e., Gomez & Carvalho, 2000), and rotifer clones were established from isolated, individually hatched eggs. As B. plicatilis belongs to a cryptic species complex, hatchlings were taxonomically identified based on restriction fragment length polymorphism (RFLP) analysis of a fragment of the mitochondrial gene COI (Campillo et al., 2005). Individual clones (30–40 per population) were maintained in the laboratory in 15 ml stock cultures and grown in a culture of the microalgae Tetraselmis suecica in artificial seawater (Instant Ocean® Sea Salt, Aquarium Systems) at 12 g l−1 salinity, f/2 enriched medium (Guillard & Ryther, 1962), and at 25°C under constant illumination (PAR: approx. 35 µEm−2 s−1). These conditions, hereafter referred to as the standard, were also used with minor modifications in the experiments. Every week, a small volume of each culture was transferred to fresh medium, and the rest was discarded.
Mating experiments
Pre-experimental cultures were established by placing five asexual, ovigerous females of each clone into a flask with 40 ml artificial seawater and T. suecica algae at a concentration of 2 × 105 cells ml−1. The aim was to achieve exponential growth, to induce sexual reproduction and to obtain a high abundance of both males and females. Thus, the flasks were checked daily until sexual reproduction was observed (i.e., when the first males were detected, after 4–7 days).
In the mating assays, young individuals (not older than 3 h) of both sexes were used to maximize the potential for mating responses. To collect virgin new-borns, eggs were detached from the female’s body by vigorous shaking of the flasks. This method has been proven efficient and harmless for eggs and hatchlings (Gomez & Serra, 1996). The eggs were sorted according to their type (smaller—male—and larger—female—eggs), collected using a micropipette, placed separately in the standard conditions with no algae addition and allowed to hatch. For each mating assay, one clone provided females (25 individuals), and a different clone provided males (six individuals). The observation protocol followed the experimental setup used in Gomez & Serra (1995), but the observation time was increased to 10 min. All the females were placed together in 50 µl fresh medium at 25°C with no algae, and males were added sequentially. After the addition of each male, the mating behaviour was observed for 10 min and then replaced with another male. This was repeated until all six males were used. The experimental volume was kept constant, as individual males were added and removed in a small volume of medium of approximately 2 µl. Observations were conducted under a stereomicroscope (Olympus SZX10, Japan), and the number of encounters, circlings and copulations was recorded according to Gomez & Serra (1995) and Snell & Hawkinson (1983). We defined an encounter as any physical contact between the male’s corona and the female’s body, a circling (implying previous encounter) was recorded when the male swam around the female body at least once, and copulation occurred when the male penetrated the female’s soft body parts (corona or foot opening). Circling always preceded copulations.
Nine population crosses were carried out for each population group (PS and LA) using males and females in all possible pairwise combinations (i.e., three intrademic and six interdemic combinations). Each population cross was replicated five times (9 crosses × 2 population groups × 5 replicates = 90 mating assays; note that six sequential measures—one per male—were obtained for each mating assay). Replication consisted of changing the clones in the mating assays. No clone was used in more than one assay within a group (5 populations × 30 clones per population = 150 clones). The experiment lasted 4 months, and one to two, rarely three, mating assays were performed per day. A single observer (I.J.) recorded the data between March and July 2018, and a previous anonymization of the experimental cultures was adopted to prevent observation bias.
Data analysis
Variation in individual male activity within an assay, albeit included in the experimental procedure, was not considered a factor in our data analysis (i.e., the counts from six males were summed up). For each population combination, we calculated the average percentage of circlings resulting from encounters (hereafter, “circling after encounter”), the average percentage of copulations resulting from circlings (“copulation after circling”) and the average percentage of copulations resulting from encounters (“copulation after encounter”), the last one being a compound of the other two.
We used a GLMM (as implemented in the lme4 package in R software, version 3.6.2) (Bates et al., 2015; R Core Team, 2019) to analyse the significance of the effects in the experimental design on copulations after encounters. Our restriction to this response was oriented to avoid dependence in our statistical analysis while favouring the biological relevance in focus. GLMM was applied separately to the PS and LA study groups. Based on the Bayesian information criterion, we assumed a binomial error distribution (assessed against a Poisson distribution with fate—copulation or not—as an additional factor). For the selected distribution, its canonical link function (logit) was used. The factors in the GLMM were (1) male-providing population (MP), (2) female-providing population (FP), (3) their interaction (MP:FP) and (4) clone pair as a random factor. Notice that the MP:FP interaction accounts for a deviation from independence (i.e., when a specific population combination deviates from the average copulations after encounters of the two populations involved). Therefore, to find a behavioural propensity against interdemic mates, a significant MP:FP interaction is necessary; however, the MP:FP effect of intrademic crosses must also be higher (i.e., relatively more copulations vs. encounters) than in interdemic crosses. Hence, to quantify this propensity between population crosses, we estimated coefficients associated with the interaction effect. These coefficients were obtained by additively decomposing the proportion of copulations (vs. encounters) observed in a mating assay. The components were (1) a (grand) mean value, (2) a component due to the male-providing population (mean deviation of that population when providing males), (3) a component due to the female-providing population (mean deviation of that population when providing females) and (4) the residual, which retains the interaction (MP:FP) and the random variation among mating assays. The decomposition follows the approach in Tortajada et al. (2009). The 45 coefficients accounting for the MP:FP interaction for each mating assay in each study group (PS or LA) were inspected to check whether the MP:FP of intrademic crosses was higher than that of interdemic crosses.
Additionally, to compare with other studies, we also computed the relative deficit–excess of interdemic copulations using a reproductive isolation index (RI, Sobel & Chen, 2014), although it presents caveats, such as that this index is affected by the indiscriminating mating activity, and mating activity might be largely sensitive to the experimental environment. RI, computed as follows:
\(1-2\frac{ \mathrm{interdemic} \,\mathrm{copulations} }{\mathrm{total \,copulations}}\) (range from − 1 to + 1) indicates the strength of the effective prezygotic barrier (opposing gene flow) in the experimental conditions.
One-sided t test on Spearman's rank correlation coefficient using the cor.test function implemented in R software, version 4.1.2. (R Core Team, 2021) was applied to assess the correlation between our mating behaviour metrics (vectors of interaction coefficients and RI) and different variables linked to the population characteristics (genetic differentiation in neutral traits, geographic distance and differentiation in environmental predictability, the latter for LA only).
Results
From the observation of the 540 males (90 mating assays; intrademic and interdemic crosses), we recorded 4,318 encounters (LA 2,055; PS 2,263), 3,191 circlings (LA 1,610; PS 1,581) and 1,850 copulations (LA 853; PS 997). Raw data from the mating experiment can be found in Supplementary Table 1. The percentages of copulations after encounters are shown in Table 3. They averaged 48% for intrademic crosses and 42% for interdemic crosses. When comparing pairwise intrademic and interdemic crosses that share a population, a higher percentage of copulations was found in intrademic crosses in 15 out of 24 comparisons. However, only SAL (in the two groups) and HYB (from the LA group) had a higher percentage of copulations for intrademic crosses in all comparisons (LA group: SAL intrademic 69.5% vs. interdemic 19.4–45.0%; HYB intrademic 56.2% vs. interdemic 19.4–50.8%, PS group: SAL intrademic 64.4% vs. interdemic 34.7–59.0%; for disaggregated values, see Table 3). In general, circling after encounters and copulation after circling had similar qualitative patterns as copulation after encounters (Supplementary Fig. 1). An exception was the CHI population (PS group), where the percentages of copulations after encounters were low and associated with high rates of encounter in intrademic crosses, while subsequent circlings and copulations did not show values remarkably different from those in other populations.
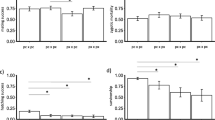
The results of the GLMM for copulation after encounter showed significant effects for all fixed factors [i.e., male-providing population (MP), female-providing population (FP), as well as its interaction (MP:FP) for each study group (Table 4)]. Figure 1 ranks the coefficients for an interaction effect obtained from additively decomposing the percentage of copulations after encounters. For both study groups, the average coefficient is higher for intrademic crosses than for interdemic crosses, indicating a higher propensity towards intrademic over interdemic crosses. This trend was stronger in the LA study group than in the PS group. The positive interaction associated with intrademic crosses is largely due to SAL and HYB populations. At the same time, in the PS study group, crosses between males from CHI and females from TON showed rather high positive values (Table 5). The prezygotic isolation index, RI, was in general slightly positive for all population combinations, pointing to weak behavioural reproductive isolation (Table 6).
Ranked coefficients associated with the interaction effect for the 45 population combinations in each study group: locally adapted (LA) and phylogeographically structured (PS) groups. The higher the value is, the higher the propensity for mating. Black bars: intrademic crosses; grey bars: interdemic crosses. The average values of intrademic and interdemic crosses are shown
Spearman’s correlation between the interaction coefficients of the two study groups and population characteristics (predictors: difference in environmental predictability, geographic distance and FST values in neutral traits) were, except for geographic distance in the PS group, significantly negative, as expected after a preference for intrademic crosses (Table 7). The correlation coefficients between RI and the predictors were positive (as expected for a preference for intrademic crosses), except for FST of microsatellites in the PS group. However, these correlations were not statistically significant.
Discussion
Our results show that populations of B. plicatilis in the Iberian Peninsula evolved a low but not negligible level of behavioural mating isolation. These populations have a deep genetic divergence associated with post-glacial recolonization (Gomez & Lunt, 2007; Campillo et al., 2011) and/or ecological divergence (Franch-Gras et al., 2017b). We documented the tendency for mating preferences using two metrics: interaction coefficients for copulations after encounters and the reproductive isolation index, RI. Both metrics are qualitatively consistent in suggesting a preference for intrademic mating. Positive interaction coefficients were mostly found for intrademic crosses, and RI had mostly positive values (five out of six comparisons), indicating a tendency for reproductive isolation. RI values are not expected to be high for populations of the same species inhabiting the same region. The RI between closely related Brachionus species ranges from 0.4 to 1.0 (Gribble & Welch, 2012), indicating incomplete behavioural isolation even between species. Overall, our results are compatible with the presence of partial behavioural isolation among our studied populations.
Despite showing the same trend, we regard our results on the interaction coefficients for successful encounters as more reliable than comparisons based on RI. Estimates of RI might be affected by differences in mating activity of the genotypes. As male–female encounters are random (Gilbert, 1963; Snell & Hawkinson, 1983), this activity depends on swimming speed, among other factors. Thus, an effect of the laboratory environment on RI is possible if, for instance, the environment favours the performance of individuals of some populations over others. Thus, following the approach in Tortajada et al. (2009), we used the interaction coefficients for encounters resulting in copulation because they are additive departures from the expectancy based on the general mating activity of the concerned genotypes. Additionally, we focused on copulations resulting from encounters because copulations have stronger implications for isolation than the other events.
Several mechanisms for behavioural mating isolation between genetically divergent populations have been recognized in the literature, namely, genetic drift acting on mating behaviour-coding genes (Kaneshiro, 1980; Carson & Templeton, 1984), indirect selection if those genes are physically linked to loci under disruptive selection and associated with local adaptation (Dobzhansky, 1937 in Gavrilets, 1999; Coyne & Orr, 2004) and direct selection if outbreeding fitness depression exists (Coyne & Orr, 2004). Conversely, when a preference for intrademic mating occurs, it should work as an intrinsic gene flow barrier enhancing genetic divergence. These mechanisms predict a negative correlation between genetic differentiation and behavioural mating. Our results show signatures of such a pattern in the two groups of populations studied here. The pattern is supported by our Spearman correlation analysis on the interaction coefficients, suggesting that, whichever the mechanisms at work are, their effects are stronger than the stabilizing selection expected to act on the mate recognition systems (Brooks et al., 2005; Smadja & Butlin, 2009), a selection whose benefit is to not become unrecognizable for a potential partner. Our results contrast with those of Berrieman et al. (2005), who, using a single clone for each of four populations, did not find evidence for reproductive isolation between the northern and southern Iberian clades of B. plicatilis.
By comparing the two experimental groups of populations, our results provide insights into the association of behavioural mating isolation with IBE and IBD. First, signatures for intrademic mating preferences are stronger in the LA group than in the PS group, despite the former populations belonging to the same phylogeographic clade (Gomez et al., 2002) and lying in geographical proximity. This suggests a stronger association of mating behaviour with IBE than with IBD and contrasts with the strong evidence for IBD in Iberian B. plicatilis populations when neutral markers were studied (Gomez et al., 2002). Second, we did not find a significant correlation between geographic distance (IBD) and mating isolation in the PS group, while in the LA group, mating isolation was slightly more correlated with environmental divergence than with geographic distance. With both predictors being collinear, the correlation with geographic distance in LA is likely spurious to some extent. As another piece of evidence, genetic differentiation in the mmr-b gene, which codes for the receptor responsible for mating recognition, increases with ecological distance (Jezkova et al., 2022).
There are several findings worthy of note. (1) The populations with the lowest differentiation in neutral traits (RAS–HYB; Montero-Pau et al., 2017) showed no mating discrimination when considering the RI index or even preference for interdemic crosses in the case of interaction coefficients. The localities of these two populations are situated very close to each other (< 1 km) and are likely to frequently merge. This suggests the role of migration in blurring differentiation. (2) Due to the historical colonization pattern, the coastal lineage and the northern lineage are the closest lineages (Gomez et al., 2000; Serra et al., 2019). This could explain why mating between TON and CHI (from the coastal and northern clades, respectively) shows lower avoidance of interdemic crosses than shown by mating between either population with SAL (the southern clade). (3) In both population groups, the SAL population drives the most distinguishable pattern of behavioural isolation, so the caveat is whether our argument is based only on one single population. We notice, however that behavioural isolation in the SAL–HYB pair is higher than that in the SAL–RAS pair, which is the expected ranking in an IBE scheme.
The association between IBE and mating isolation found herein makes the question of selection of isolation (Butlin, 1987) worthy of investigation in these rotifers. An important condition for selection for premating isolation is that the intensity of the potential gene flow must be intermediate, meeting a balance between the homogenizing effect of extensive gene flow and lack of gene flow (no need for selection for isolation; (Nosil, 2013; Yukilevich, 2012). Even if direct measures of migration rates for rotifers are scarce (Lopes et al., 2016; Moreno et al., 2019), a gradient of rates is most likely in populations such as those in the LA group.
In our research, we focused on behavioural mating isolation as a premating (i.e., low-cost) intrinsic barrier. As anticipated above, other intrinsic barriers not captured here might be involved in premating isolation in the wild. In Brachionus rotifers, the beginning of sexual reproduction is triggered by the accumulation of a crowding chemical signal in the environment (Snell et al., 2006; Snell, 2017). While it was shown that the signal is rather conservative and sex can be cross-induced between species (Stelzer & Snell, 2006; García-Roger et al., 2009), the threshold concentration varies even among genotypes of a single species (Franch-Gras et al., 2017a). These differences could affect the timing of sex, thereby promoting reproductive isolation between immigrants and residents via their allochronic sexual periods.
How populations differentiate, become locally adapted and eventually diverge into distinct species are persistent questions in evolutionary biology, and their answer depends upon a panoply of processes acting in opposing directions, with complex interactions due to causal feedbacks and having different strengths in relation to organisms and habitat features. The taxon of cyclically parthenogenetic rotifers harbours high species richness, and the few well-studied species in the taxon are clusters of genetically differentiated and locally adapted populations. Our results suggest that isolation may arise associated with genetic divergence and might play a role in the diversification of rotifers. We nevertheless remark that behavioural isolation is just a piece of the premating isolation puzzle.
Data availability
Enquiries about data availability should be directed to the authors.
Change history
14 July 2022
A Correction to this paper has been published: https://doi.org/10.1007/s10750-022-04946-1
References
Arthur, N. J. & K. A. Dyer, 2015. Asymmetrical sexual isolation but no postmating isolation between the closely related species Drosophila suboccidentalis and Drosophila occidentalis. BMC Evolutionary Biology 15: 38–46.
Bates, D., M. Mächler, B. Bolker & S. Walker, 2015. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67: 1–48.
Berrieman, H. K., D. H. Lunt & A. Gomez, 2005. Behavioural reproductive isolation in a rotifer hybrid zone. Hydrobiologia 546: 125–134.
Bohonak, A. J. & D. G. Jenkins, 2003. Ecological and evolutionary significance of dispersal by freshwater invertebrates. Ecology Letters 6: 783–796.
Brooks, R., J. Hunt, M. W. Blows, M. J. Smith, L. F. Bussière & M. D. Jennions, 2005. Experimental evidence for multivariate stabilizing sexual selection. Evolution 59: 871–880.
Butlin, R., 1987. Speciation by reinforcement. Trends in Ecology and Evolution 2: 8–13.
Campillo, S., E. M. García-Roger, D. Martínez-Torres & M. Serra, 2005. Morphological stasis of two species belonging to the L-morphotype in the Brachionus plicatilis species complex. Hydrobiologia 546: 181–187.
Campillo, S., M. Serra, M. J. Carmona & A. Gomez, 2011. Widespread secondary contact and new glacial refugia in the halophilic rotifer Brachionus plicatilis in the Iberian Peninsula. PLoS ONE 6: e20986.
Carranza, J., M. Roldán, E. F. C. de Peroni & J. M. B. Duarte, 2017. Weak premating isolation between two parapatric brocket deer species. Mammalian Biology 87: 17–26.
Carson, H. L. & A. R. Templeton, 1984. Genetic revolutions in relation to speciation phenomena: the founding of new populations. Annual Review of Ecology and Systematics 15: 97–132.
Castro Vargas, C., M. P. Richmond, M. Ramirez Loustalot Laclette & T. A. Markow, 2017. Early events in speciation: cryptic species of Drosophila aldrichi. Ecology and Evolution 7: 4220–4228.
Church, S. A. & D. R. Taylor, 2002. The evolution of reproductive isolation in spatially structured populations. Evolution 56: 1859–1862.
Cowles, S. A. & J. A. C. Uy, 2019. Rapid, complete reproductive isolation in two closely related Zosterops White-eye bird species despite broadly overlapping ranges. Evolution 73: 1647–1662.
Coyne, J. A. & H. A. Orr, 2004. Speciation, Sinauer Associates, Sunderland:
Cruz-Yepez, N., C. González & J. F. Ornelas, 2020. Vocal recognition suggests premating isolation between lineages of a lekking hummingbird. Behavioral Ecology 31: 1046–1053.
De Meester, L., A. Gomez, B. Okamura & K. Schwenk, 2002. The Monopolization Hypothesis and the dispersal-gene flow paradox in aquatic organisms. Acta Oecologica 23: 121–135.
Declerck, S. A. J. & S. Papakostas, 2016. Monogonont rotifers as model systems for the study of micro-evolutionary adaptation and its eco-evolutionary implications. Hydrobiologia 796: 131–144.
Declerck, S., C. Cousyn & L. De Meester, 2001. Evidence for local adaptation in neighbouring Daphnia populations: a laboratory transplant experiment. Freshwater Biology 46: 187–198.
Franch-Gras, L., E. M. García-Roger, B. Franch, M. J. Carmona & M. Serra, 2017a. Quantifying unpredictability: a multiple-model approach based on satellite imagery data from Mediterranean ponds. PLoS ONE 12: e0187958.
Franch-Gras, L., E. M. García-Roger, M. Serra & M. José Carmona, 2017b. Adaptation in response to environmental unpredictability. Proceedings of the Royal Society B: Biological Sciences 284: 20170427.
Franch-Gras, L., C. Hahn, E. M. García-Roger, M. J. Carmona, M. Serra & A. Gomez, 2018. Genomic signatures of local adaptation to the degree of environmental predictability in rotifers. Scientific Reports 8: 1–14.
García-Roger, E. M., N. Dias, M. J. Carmona & M. Serra, 2009. Crossed induction of sex in sympatric congeneric rotifer populations. Limnology and Oceanography 54: 1845–1854.
Gavrilets, S., 1999. A dynamical theory of speciation on holey adaptive landscapes. American Naturalist 154: 1–22.
Gilbert, J. J., 1963. Contact chemoreception, mating behaviour, and sexual isolation in the rotifer genus Brachionus. Journal of Experimental Biology 40: 625–641.
Gomez, A. & G. R. Carvalho, 2000. Sex, parthenogenesis and genetic structure of rotifers: microsatellite analysis of contemporary and resting egg bank populations. Molecular Ecology 9: 203–214.
Gomez, A. & D. H. Lunt, 2007. Refugia within Refugia: Patterns of Phylogeographic Concordance in the Iberian Peninsula Phylogeography of Southern European Refugia: Evolutionary Perspectives on the Origins and Conservation of European Biodiversity, Springer, Dordrecht:, 155–188.
Gomez, A. & M. Serra, 1995. Behavioral reproductive isolation among sympatric strains of Brachionus plicatilis Müller 1786: insights into the status of this taxonomic species. Hydrobiologia 313–314: 111–119.
Gomez, A. & M. Serra, 1996. Mate choice in male Brachionus plicatilis rotifers. Functional Ecology 10: 681.
Gomez, A., G. R. Carvalho & D. H. Lunt, 2000. Phylogeography and regional endemism of a passively dispersing zooplankter: mitochondrial DNA variation in rotifer resting egg banks. Proceedings of the Royal Society B: Biological Sciences 267: 2189–2197.
Gomez, A., G. J. Adcock, D. H. Lunt & G. R. Carvalho, 2002. The interplay between colonization history and gene flow in passively dispersing zooplankton: microsatellite analysis of rotifer resting egg banks. Journal of Evolutionary Biology 15: 158–171.
Gribble, K. E. & D. B. M. Welch, 2012. The mate recognition protein gene mediates reproductive isolation and speciation in the Brachionus plicatilis cryptic species complex. BMC Evolutionary Biology 12: 134–151.
Gribble, K. E., T. W. Snell & D. B. M. Welch, 2011. Gene and protein structure of the mate recognition protein gene family in Brachionus manjavacas (Rotifera). Hydrobiologia 662: 35–42.
Guillard, R. R. & J. H. Ryther, 1962. Studies of marine planktonic diatoms. I. Cyclotella nana Hustedt, and Detonula confervacea (cleve) Gran. Canadian Journal of Microbiology 8: 229–239.
Hawthorne, D. J. & S. Via, 2001. Genetic linkage of ecological specialization and reproductive isolation in pea aphids. Nature 412: 904–907.
Ismail, M. & M. Brooks, 2018. Male mating preference of two cryptic species of the herbivorous insect Eccritotarsus catarinensis. Biocontrol Science and Technology 28: 529–543.
Jezkova, I., M. Serra, R. Ortells & J. Montero, 2022. Genetic variability of the mating recognition gene in populations of Brachionus plicatilis. Diversity 14: 155.
Jiggins, C. D., C. Estrada & A. Rodrigues, 2004. Mimicry and the evolution of premating isolation in Heliconlus melpomene Linnaeus. Journal of Evolutionary Biology 17: 680–691.
Juárez, M. L., F. Devescovi, R. Břízová, G. Bachmann, D. F. Segura, B. Kalinová, P. Fernández, M. J. Ruiz, J. Yang, P. E. A. Teal, C. Cáceres, M. J. B. Vreysen, J. Hendrichs & M. T. Vera, 2015. Evaluating mating compatibility within fruit fly cryptic species complexes and the potential role of sex pheromones in pre-mating isolation. ZooKeys 540: 125–155.
Kaneshiro, K. Y., 1980. Sexual isolation, speciation and the direction of evolution. Evolution 34: 437–444.
Langton-Myers, S. S., G. I. Holwell & T. R. Buckley, 2019. Weak premating isolation between Clitarchus stick insect species despite divergent male and female genital morphology. Journal of Evolutionary Biology 32: 398–411.
Lopes, P. M., R. Bozelli, L. M. Bini, J. M. Santangelo & S. A. J. Declerck, 2016. Contributions of airborne dispersal and dormant propagule recruitment to the assembly of rotifer and crustacean zooplankton communities in temporary ponds. Freshwater Biology 61: 658–669.
Louette, G. & L. De Meester, 2005. High dispersal capacity of cladoceran zooplankton in newly founded communities. Ecology 86: 353–359.
Luo, Z. X., Z. Q. Li, X. M. Cai, L. Bian & Z. M. Chen, 2017. Evidence of premating isolation between two sibling moths: Ectropis grisescens and Ectropis obliqua (Lepidoptera: Geometridae). Journal of Economic Entomology 110: 2364–2370.
Maltseva, A. L., M. A. Varfolomeeva, A. A. Lobov, P. O. Tikanova, E. A. Repkin, I. Y. Babkina, M. Panova, N. A. Mikhailova & A. I. Granovitch, 2021. Premating barriers in young sympatric snail species. Scientific Reports 11: 1–16.
Martin, G. K., B. E. Beisner, F. J. J. Chain, M. E. Cristescu, P. A. Giorgio & A. M. Derry, 2021. Freshwater zooplankton metapopulations and metacommunities respond differently to environmental and spatial variation. Ecology, Ecological Society of America 102: e03224.
Meguro, Y., H. Takahashi, Y. Machida, H. Shirakawa, M. R. Gaither & A. Goto, 2016. Assortative mating and divergent male courtship behaviours between two cryptic species of nine-spined sticklebacks (genus Pungitius). Behaviour 153: 1879–1911.
Mills, S., D. H. Lunt & A. Gomez, 2007. Global isolation by distance despite strong regional phylogeography in a small metazoan. BMC Evolutionary Biology 7: 225.
Montero-Pau, J., M. Serra & A. Gomez, 2017. Diapausing egg banks, lake size, and genetic diversity in the rotifer Brachionus plicatilis Müller (Rotifera, Monogononta). Hydrobiologia 796: 77–91.
Moreno, E., C. Pérez-Martínez & J. M. Conde-Porcuna, 2019. Dispersal of rotifers and cladocerans by waterbirds: seasonal changes and hatching success. Hydrobiologia 834: 145–162.
Nosil, P., 2013. Degree of sympatry affects reinforcement in Drosophila. Evolution 67: 868–872.
Nosil, P., B. J. Crespi & C. P. Sandoval, 2002. Host-plant adaptation drives the parallel evolution of reproductive isolation. Nature 417: 440–443.
Nosil, P., T. H. Vines & D. J. Funk, 2005. Perspective: reproductive isolation caused by natural selection against immigrants from divergent habitats. Evolution 59: 705.
Palumbi, S. R., 1994. Genetic divergence, reproductive isolation, and marine speciation. Annual Review of Ecology and Systematics 25: 547–572.
Parchman, T. L., Z. Gompert, M. J. Braun, R. T. Brumfield, D. B. McDonald, J. A. C. Uy, G. Zhang, E. D. Jarvis, B. A. Schlinger & C. A. Buerkle, 2013. The genomic consequences of adaptive divergence and reproductive isolation between species of manakins. Molecular Ecology 22: 3304–3317.
R Core Team, 2019. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna: Austria.
R Core Team, 2021. R: A Language and Environment for Statistical Computing, R Foundation for Statistical Computing, Vienna: Austria.
Schröder, T. & E. J. Walsh, 2007. Cryptic speciation in the cosmopolitan Epiphanes senta complex (Monogononta, Rotifera) with the description of new species. Hydrobiologia 593: 129–140.
Schröder, T. & E. J. Walsh, 2010. Genetic differentiation, behavioural reproductive isolation and mixis cues in three sibling species of Monogonont rotifers. Freshwater Biology 55: 2570–2584.
Serra, M., T. W. Snell & R. L. Wallace, 2018. Reproduction, overview by phylogeny: Rotifera. In Encyclopedia of Reproduction. Elsevier, Amsterdam: 513–521.
Serra, M., E. M. García-Roger, R. Ortells & M. J. Carmona, 2019. Cyclically parthenogenetic rotifers and the theories of population and evolutionary ecology. Limnetica 38: 67–93.
Sexton, J. P., S. B. Hangartner & A. A. Hoffmann, 2014. Genetic isolation by environment or distance: which pattern of gene flow is most common? Evolution 68: 1–15.
Shafer, A. B. A. & J. B. W. Wolf, 2013. Widespread evidence for incipient ecological speciation: a meta-analysis of isolation-by-ecology. Ecology Letters 16: 940–950.
Smadja, C. & R. K. Butlin, 2009. On the scent of speciation: the chemosensory system and its role in premating isolation. Heredity 102: 77–97.
Snell, T. W., 2017. Analysis of proteins in conditioned medium that trigger monogonont rotifer mictic reproduction. Hydrobiologia 796: 245–253.
Snell, T. W. & E. M. Boyer, 1988. Thresholds for mictic female production in the rotifer Brachionus plicatilis (Muller). Journal of Experimental Marine Biology and Ecology 124: 73–85.
Snell, T. W. & M. Childress, 1987. Aging and loss of fertility in male and female Brachionus plicatilis (rotifera). International Journal of Invertebrate Reproduction and Development 12: 103–110.
Snell, T. W. & B. L. Garman, 1986. Encounter probabilities between male and female rotifers. Journal of Experimental Marine Biology and Ecology 97: 221–230.
Snell, T. W. & C. A. Hawkinson, 1983. Behavioral reproductive isolation among populations of the rotifer Brachionus plicatilis. Evolution 37: 1294–1305.
Snell, T. W. & F. H. Hoff, 1987. Fertilization and male fertility in the rotifer Brachionus plicatilis. Hydrobiologia 147: 329–334.
Snell, T. W. & P. D. Morris, 1993. Sexual communication in copepods and rotifers. Hydrobiologia 255–256: 109–116.
Snell, T. W., R. Rico-Martinez, L. N. Kelly & T. E. Battle, 1995. Identification of a sex pheromone from a rotifer. Marine Biology 123: 347–353.
Snell, T. W., J. Kubanek, W. Carter, A. B. Payne, J. Kim, M. K. Hicks & C. P. Stelzer, 2006. A protein signal triggers sexual reproduction in Brachionus plicatilis (Rotifera). Marine Biology 149: 763–773.
Snell, T. W., J. Kim, E. Zelaya & R. Resop, 2007. Mate choice and sexual conflict in Brachionus plicatilis (Rotifera). Hydrobiologia 593: 151–157.
Sobel, J. M. & G. F. Chen, 2014. Unification of methods for estimating the strength of reproductive isolation. Evolution 68: 1511–1522.
Sobel, J. M. & M. A. Streisfeld, 2015. Strong premating reproductive isolation drives incipient speciation in Mimulus aurantiacus. Evolution 69: 447–461.
Stelzer, C. P. & T. W. Snell, 2003. Induction of sexual reproduction in Brachionus plicatilis (Monogononta, Rotifera) by a density-dependent chemical cue. Limnology and Oceanography 48: 939–943.
Stelzer, C. P. & T. W. Snell, 2006. Specificity of the crowding response in the Brachionus plicatilis species complex. Limnology and Oceanography 51: 125–130.
Tortajada, A. M., M. J. Carmona & M. Serra, 2009. Does haplodiploidy purge inbreeding depression in rotifer populations? PLoS ONE 4: e8195.
Turbek, S. P., E. S. C. Scordato & R. J. Safran, 2018. The role of seasonal migration in population divergence and reproductive isolation. Trends in Ecology and Evolution 33: 164–175.
Turbek, S. P., M. Browne, A. S. Di Giacomo, C. Kopuchian, W. M. Hochachka, C. Estalles, D. A. Lijtmaer, P. L. Tubaro, L. F. Silveira, I. J. Lovette, R. J. Safran, S. A. Taylor & L. Campagna, 2021. Rapid speciation via the evolution of pre-mating isolation in the Iberá Seedeater. Science 371: eabc0256.
Ventura, M., A. Petrusek, A. Miró, E. Hamrová, D. Buñay, L. De Meester & J. Mergeay, 2014. Local and regional founder effects in lake zooplankton persist after thousands of years despite high dispersal potential. Molecular Ecology 23: 1014–1027.
Wallace, R. L., T. W. Snell & H. A. Smith, 2015. Phylum Rotifera. In Thorp, J. & D. C. Rogers (eds), Ecology and General Biology: Thorp and Covich’s Freshwater Invertebrates Academic, New York: 225–271.
Wang, I. J. & G. S. Bradburd, 2014. Isolation by environment. Molecular Ecology 23: 5649–5662.
Weber, M. G. & S. Y. Strauss, 2016. Coexistence in close relatives: beyond competition and reproductive isolation in sister taxa. Annual Review of Ecology, Evolution, and Systematics 47: 359–381.
Weber, J. N., G. S. Bradburd, Y. E. Stuart, W. E. Stutz & D. I. Bolnick, 2017. Partitioning the effects of isolation by distance, environment, and physical barriers on genomic divergence between parapatric three-spine stickleback. Evolution 71: 342–356.
Wright, S., 1943. Isolation by distance. Genetics 28: 114–138.
Wu, C.-I., 2001. The genic view of the process of speciation. Journal of Evolutionary Biology 14: 851–865.
Yukilevich, R., 2012. Asymmetrical patterns of speciation uniquely support reinforcement in Drosophila. Evolution 66: 1430–1446.
Zeller, M., T. B. H. Reusch & W. Lampert, 2006. A comparative population genetic study on calanoid freshwater copepods: Investigation of isolation-by-distance in two Eudiaptomus species with a different potential for dispersal. Limnology and Oceanography 51: 117–124.
Acknowledgements
We thank Eduardo M. García-Roger for his helpful comments on the data analysis.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The research was supported by a Grant from the Valencian Conselleria de Educación, Investigación, Cultura y Deporte (AICO/2020/013), a Grant CGL2015-65422-P funded by MCIN/AEI/10.13039/501100011033 and by ERDF A way of making Europe. IJ was supported by a Grant from the Valencian Consellería de Educación, Investigación, Cultura y Deporte (GRISOLIAP/2017/096).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflicts of interest.
Additional information
Handling Editor: Diego Fontaneto
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original version of this article was revised: one of the grant numbers contained an error and should read: MCIN/AEI/10.13039/501100011033.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jezkova, I., Ortells, R., Montero-Pau, J. et al. Insight into incipient reproductive isolation in diverging populations of Brachionus plicatilis rotifer. Hydrobiologia 849, 3299–3311 (2022). https://doi.org/10.1007/s10750-022-04927-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10750-022-04927-4