Abstract
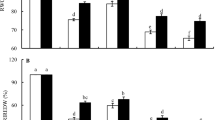
Plasma membrane (PM) vesicles were isolated from developing embryos of wheat (Triticum aestivum L.) with different drought-tolerance under drought stress by the gradient centrifugation method. The activity of the PM H+-ATPase (EC 3.6.1.35) and contents of polyamine conjugated (covalently and noncovalently) to the PM vesicles were investigated. Results showed that after drought treatment for 3 d, embryo relative water content (ERWC), embryo relative dry weight increase rate (ERDWIR) of drought-sensitive Yumai No. 48 cultivar decreased more significantly than those of drought-tolerant Luomai No. 22 cultivar, while PM H+-ATPase activity, noncovalently conjugated (NCC) spermidine (Spd) and NCC spermine (Spm), the covalently conjugated (CC) putrescine (Put) and CC Spd of PM from Luomai No. 22 cultivar increased more obviously than those from Yumai No. 48 cultivar. As judged by increases in ERWC and ERDWIR, treatment with exogenous Spd alleviated markedly drought injuries to Yumai No. 48, coupled with significant increases in NCC Spd and NCC Spm levels and H+-ATPase activity in the embryo PM vesicle. Under drought stress, the treatment of drought-tolerant Luomai No. 22 cultivar with methylglyoxyl-bis (guanylhydrazone) (MGBG), an inhibitor of S-adenosylmethionine decarboxylase (SAMDC), and phenanthrolin (o−Phen), an inhibitor of transglutaminase (TGase) respectively, caused a decrease of the NCC Spd, NCC Spm, CC Put and CC Spd. Those decreases were associated with decreased PM-H+-ATPase activity and the tolerance of developing wheat embryos to osmotic stress, as judged by decreases in ERWC and ERDWIR. These results suggest that tolerance of the developing wheat embryos to drought stress is associated with the embryo PM H+-ATPase and the levels of NCC Spd, NCC Spm, CC Put and CC Spd in embryo PM vesicles.







Similar content being viewed by others
Abbreviations
- BSA:
-
Bovine serum albumin
- CC:
-
Covalently conjugated
- DR:
-
Drought
- DTT:
-
Dithiothreitol
- EGTA:
-
Ethylene glycol tetraacetic acid
- ERDWIR:
-
Embryo relative dry weight increase rate
- ERWC:
-
Embryo relative water content
- Hepes:
-
2-[4-(2-Hydroxyethyl)-1-piperazinyl]ethanesulfonic acid
- HPLC:
-
High performance liquid chromatography
- MGBG:
-
Methylglyoxyl-bis (guanylhydrazone)
- NCC:
-
Noncovalently conjugated
- O−Phen:
-
Phenanthrolin
- PA:
-
Polyamine
- PCA:
-
Perchloric acid
- PEG:
-
Polyethylene glycol–6000
- PM:
-
Plasma membrane
- PMSF:
-
Phenylmethanesulfonyl fluoride
- Put:
-
Putrescine
- PVP:
-
Polyvinyl pyrrolidone
- SAMDC:
-
S-adenosylmethionine decarboxylase
- Spd:
-
Spermidine
- Spm:
-
Spermine
- TCA:
-
Trichloroacetic acid
- TGase:
-
Transglutaminase
References
Adiga PR, Prasad GL (1985) Biosynthesis and regulation of polyamines in higher plants. Plant Growth Regul 3:205–226
Astarita LV, Handro W, Floh ES (2003) Changes in polyamines content associated with zygotic embryogenesis in the Brazilian pine, Araucaria angustifolia (Bert.) O. Ktze. Rev Bras Bot 26(2):163–168
Athwal GS, Huber SC (2002) Divalent cations and polyamines bind to loop-8 of 14-3-3 proteins, modulating their interaction with phosphorylated nitrate reductase. Plant J 29:119–129
Bradford MM (1976) A rapid and sensitive methods for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binging. Anal Biochem 72:248–254
Cacho M, Torres DA, Elena-Rosselló JA (2013) Role of polyamines in regulating silymarin production in Silybum marianum (L.) Gaertn (Asteraceae) cell cultures under conditions of calcium deficiency. J Plant Physiol 170(15):1344–1348
Camoni L, Lucente CD, Pallucca R, Visconti S, Aducci P (2012) Binding of phosphatidic acid to 14-3-3 proteins hampers their ability to activate the plant plasma membrane H+-ATPase. IUBMB Life 64:710–716
Cao DD, Hu J, Zhu SJ, Hu WM, Knapp A (2010) Realtionship between changes in endogenous polyamines and seed quality during development of sh 2 sweet corn (Zea mays L.) seed. Sci Horti 123:301–307
Del Duca S, Beninati S, Serafini-Fracassini D (1995) Polyamines in chloroplasts: identification of their glutamyl and acetyl derivatives. Biochem J 305:233–237
Di Tomaso JM, Shaff JE, Kochian LV (1989) Putrescine-induced wounding and its effects on membrane integrity and ion transport processes in roots of intact corn seedlings. Plant Physiol 90:988–995
Do PT, Degenkolbe T, Erban A, Heyer AG, Kopka J, Kohl KL, Hincha DK, Zuther E (2013) Dissecting rice polyamine metabolism under controlled long-term drought stress. PLoS ONE 8(4):e60325. doi:10.1371/journal.pone.0060325
Dutra NT, Silveira V, Azevedo IG, Gomes-Neto LR, Facanha AR, Steiner N, Guerra MP, Floh EIS, Santa-Catarina C (2013) Polyamines affect the cellular growth and structure of pro-embryogenic masses in Araucaria angustifolia embryogenic cultures through the modulation of proton pump activities and endogenous levels of polyamines. Physiol Plant 148:121–132
Farooq M, Wahid A, Lee DJ (2009) Exogenously applied polyamines increase drought tolerance of rice by improving leaf water status, photosynthesis and membrane properties. Acta Physiol Plant 31:937–945
Galston AW, Kaur-Sawhney R (1995) Polyamines as endogenous growth regulators. In: Davies PJ (ed) Plant Hormones: Physiology. Dordrecht, Kluwer Academic Publishers, Biochemistry and Molecular Biology, pp 158–178
Garufi A, Visconti S, Camoni L, Aducci P (2007) Polyamines as physiological regulators of 14-3-3 interaction with the plant plasma membrane H+-ATPase. Plant Cell Physiol 48:434–440
Goyal M, Asthir B (2010) Polyamine catabolism influences antioxidative defense mechanism in shoots and roots of five wheat genotypes under high temperature stress. Plant Growth Regul 60:13–25
Grzesiak M, Filek M, Barbasz A, Kreczmer B, Hartikainen H (2013) Relationships between polyamines, ethylene, osmoprotectants and antioxidant enzymes activities in wheat seedlings after short-term PEG- and NaCl-induced stresses. Plant Growth Regul 69:177–189
Gupta K, Dey A, Gupta B (2013) Plant polyamines in abiotic stress responses. Acta Physiol Plant 35:2015–2036
Iqbal M, Ashraf M (2005) Changes in growth, photosynthetic capacity and ionic relations in spring wheat (Triticum aestivum L.) due to pre-sowing seed treatment with polyamines. Plant Growth Regul 46:19–30
Janicka-Russak M, Kabala K, Mlodzinska E, Klobus G (2010) The role of polyamines in the regulation of the plasma membrane and the tonoplast proton pumps under salt stress. J Plant Physiol 167:261–269
Jelich OC, Weiler EW, Oeching C (2001) Binding of regulatory 14-3-3 proteins to the C-terminus of the plant plasma membrane H+-ATPase involves part of its autoinhibitory region. J Biol Chem 276:39852–39857
Johansson F, Sommarin M, Larsson C (1993) Fusicoccin activates the plasma membrane H+-ATPase by a mechanism involving the C-terminal inhibitory domain. Plant Cell 5:321–327
Kaur-Sawhney R, Tiburcio AF, Atabella T, Galston AW (2003) Polyamines in plants: an overview. J Cell Mol Biol 2:1–12
Kinoshita T, Nishimura M, Shimazaki K (1995) Cytosolic concentration of C 2+a regulates the plasma membrane H+-ATPase in guard cells of fava bean. Plant Cell 7:1333–1342
Kubis J (2008) Exogenous spermidine differentially alters activities of some scavenging system enzymes, H2O2 and superoxide radical levels in water-stressed cucumber leaves. J Plant Physiol 165:397–406
Kumer A, Altabella T, Taylor MA, Tiburcio AF (1997) Recent advances in polyamine research. Trends Plant Sci 2:124–130
Legocka J, Sobieszczuk-Nowicka E (2012) Sorbitol and NaCl stresses affect free, microsome-associated and thylakoid-associated polyamine content in Zea mays and Phaseolus vulgaris. Acta Physiol Plant 34:1145–1151
Lester GE (2000) Polyamines and their cellular anti-senescence properties in honeydew muskmelon fruit. Plant Sci 160:105–112
Li XN, Jiang HD, Liu FL, Cai J, Dai TB, Cao WX, Jiang D (2013) Induction of chilling tolerance in wheat during germination by pre-soaking seed with nitric oxide and gibberellin. Plant Growth Regul 71:31–40
Liu HP, Dong BH, Zhang YY, Liu ZP, Liu YL (2004) Relationship between osmotic stress and the levels of free, conjugated and bound Polyamines in leaves of wheat seedlings. Plant Sci 166:1261–1267
Liu J, Yu BJ, Liu YL (2006) Effects of spermidine and spermine levels on salt tolerance associated with tonoplast H+-ATPase and H+-PPase activities in barley roots. Plant Growth Regul 49:119–126
Martin-Tanguy J (2001) Metabolism and function of polyamines in plants: recent development (new approaches). Plant Growth Regul 34:135–148
Michelet B, Boutry M (1995) The plasma membrane H+-ATPase: a highly regulated enzyme with multiple physiological functions. Plant Physiol 108:1–6
Michelet B, Lukaszewiez M, Dupriez V, Boutry M (1994) A plant plasma membrane proton-ATPase gene is regulated by development and environment and shows signs of a translational regulation. Plant Cell 6:1375–1389
Niemenak N, Awah TM, Lieberei R (2012) Establishment of suspension culture in Theobroma cacao and polyamines associated with cacao embryogenesis. Plant Growth Regul 67:1–8
Ohinishi T, Gall RG, Mayer ML (1975) An improved assay of inorganic phosphate in the presence of extralabile phosphate compounds: application to the ATPase assay in the presence of phosphocreatine. Anal Biochem 689:261–267
Palmgren MG, Sommarin M, Serrano R, Larson C (1991) Identification of an autoinhibitory domain in the C-terminal region of the plant plasma membrane H+-ATPase. J Biol Chem 266:20470–20475
Peng YB, Lu YF, Zhang DP (2003) Abscisic acid activates ATPase in developing apple fruit especially in fruit phloem cells. Plant, Cell Environ 26:1329–1342
Qiao XQ, Shi GX, Jia R, Chen L, Tian XL, Xu J (2012) Physiological and biochemical responses induced by lead stress in Spirodela polyrhiza. Plant Growth Regul 67:217–225
Qiu QS, Su XF (1998) The influence of extracellular-side Ca2+ on the activity of the plasma membrane H+-ATPase from wheat roots. Aust J Plant Physiol 25:923–928
Qiu QS, Zhang N (2000) Water stress inhibits P-nitrophenyl phosphate hydrolysis activity of the plasma membrane H+-ATPase from soybean hypocotyls. Aust J Plant Physiol 27:717–721
Reggiani R, Zaina S, Bertani A (1992) Plasma membrane ATPase in rice coleoptiles: stimulation by putrescine and polyamines. Phytochemistry 31:417–419
Sailerova E, Zwiazek JJ (1993) Effects of triadimefon and osmotic stress on the plasma membrane composition and ATPase activity in the white spruce (Picea Glauca) needles. Physiol Plant 87:475–482
Schonfeld MA, Johnson RC, Carver BF, Mornhinwey DW (1988) Water relation in winter wheat as drought resistance indicators. Crop Sci 28:526–537
Serrano R (1989) Structure and function of plasma membrane H+-ATPase. Annu Rev Plant Physiol Plant Mol Biol 40:61–94
Sharma P, Rajam MV (1995) Spatial and temporal changes in endogenous polyamine levels associated with osmotic embryogenesis from different hypocotyls segments of eggplant (Solanum melongena L.). J Plant Physiol 146:658–664
Shen W, Huber SC (2006) Polycations globally enhance binding of 14-3-3ω to target proteins in spinach leaves. Plant Cell Physiol 47:764–771
Slocum RD (1991) Polyamine biosynthesis in plant. In: Slocum RD, Flores HE (eds) Polyamines in Plants. CRC Press, Florida, pp 23–40
Sood S, Nagar PK (2003) The effect of polyamines on leaf senescence in two diverse rose species. Plant Growth Regul 39:155–160
Srivastava SK, Rajbabu P (1983) Effect of amines and guanidines on ATPase from maize scutellum. Phytochemistry 22:2675–2679
Surowy TK, Boyer JS (1991) Low water potentials affect expression of genes encoding vegetative storage proteins and plasma membrane protein ATPase in soybean. Plant Mol Biol 16:251–262
Sussman MR (1994) Molecular analysis of protein in the plasma membrane. Annu Rev Plant Physiol Plant Mol Biol 45:213–234
Sussman MR, Harper JF (1989) Molecular biology of the plasma membrane of higher plants. Plant Cell 1:953–960
Suwastika IN, Gehring CA (1999) The plasma membrane H+-ATPase from Tradescantia stem and leaf tissue is modulated in vitro by Cgmp. Arch Biochem Biophys 367:137–139
Svennelid F, Olsson A, Piotrowski M, Rosenquist M, Ottman C, Larsson C, Oecking C, Sommarin M (1999) Phosphorylation of Thr-948 at the C-terminus of the plasma membrane H+-ATPase creats a binding site for the regulatory 14-3-3 protein. Plant Cell 11:2379–2391
Tang W, Newton RJ (2005) Polyamines reduce salt-induced oxidative damage by increasing the activities of antioxidant enzymes and decreasing lipid peroxidation in Virginia pine. Plant Growth Regul 46:31–43
Tiburcio AF, Campos JL, Figueras X (1993) Recent advances in the understanding of polyamines functions during plant development. Plant Growth Regul 12:331–340
Uranoa K, Hoboa T, Shinozaki K (2005) Arabidopsis ADC genes involved in polyamine biosynthesis are essential for seed development. FEBS Lett 579:1557–1564
Widell S, Larsson C (1990) A critical evaluation of markers used in plasma membrane purification. In: Larsson C, Moller IM (eds) The Plant Plasma Membrane. Springer-Verley, Berlin, pp 16–43
Yamaguchi K, Takahashi Y, Berberich T, Imai A, Takahashi T, Michael AJ, Kusano T (2007) A protective role for the polyamine spermine against drought stress in Arabidopsis. Biochem Biophys Res Commun 352:486–490
Zepeda-Jazo I, Velarde-Buendia AM, Enriquez-Figueroa R, Bose J, Shabala S, Muniz-Murguia J, Pottosin II (2011) Polyamines interact with hydroxyl radicals in activating Ca2+ and K+ transport across the root epidermal plasma membrane. Plant Physiol 157:2167–2180
Zhang WH, Chen Q, Liu YL (2002) Relationship between H+-ATPase activity and fluidity of tonoplast in barley roots under NaCl stress. Acta Botanic Sinic 44:292–296
Zhao FG, Sun C, Liu YL, Liu ZP (2000) Effect of salinity stress on the levels of covalently and noncovalently conjugated polyamines in plasma membrane and tonoplast isolated from barley seedlings. Acta Botanic Sinic 42:920–926
Acknowledgments
This research was supported by the National Natural Science Foundation of China (Grant No. 31271627), the Special Fund of Industrial (Agriculture) Research for Public Welfare of China (Grant No. 201203077) and the Education Department Natural Science Foundation of Henan Province (Grant No. 2011A180033).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Du, H., Zhou, X., Yang, Q. et al. Changes in H+-ATPase activity and conjugated polyamine contents in plasma membrane purified from developing wheat embryos under short-time drought stress. Plant Growth Regul 75, 1–10 (2015). https://doi.org/10.1007/s10725-014-9925-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10725-014-9925-9