Abstract
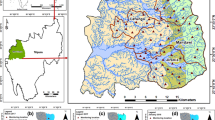
Due to water scarcity, the groundwater will represent an essential source of water in many communities worldwide. This study was carried out to investigate the main hydrogeochemical characteristic of trace elements composition, their sources, and its vulnerability in groundwater to the human population. Fifteen groundwater samples were collected from boreholes and hand dug wells from the South Eastern Desert, Egypt, and analyzed for Al, As, B, Fe, Mn, Cd, Co, Cr, Cu, Hg, Ni, Pb, Rb, Sb, Sr, Th, U, V, and Zn using inductively coupled plasma mass spectrometry. Multivariate analyses were applied to identify the distribution and potential source of trace elements. The groundwater is tapped from the Miocene and the fractured basement rock aquifers. The mean concentrations of trace elements exceed the guideline values of all organizations, except in some wells for Zn, Cu, and Co. Cationic trace elements declined in the order of Mn > Fe > Zn > Al > V > Ni > Rb > Sr > U > Cu > Cr > Co > Cd > Pb > Th > Sb > Hg. Oxyanions As (mean 15.48 mg/L) and B (mean 1.24 mg/L) showed very high concentrations and higher than the average WHO concentrations in water suggesting potential adverse toxicity to all aquatic organisms. Five factor analyses indicated that different geochemical contributions are involved in the chemical characteristics of groundwater in the study area. Water–rock interaction and dissolution processes in bed rocks from different coastal Miocene deposits, meta-volcanics, basic-ultrabasic rocks, granitic and meta-sediments, seawater intrusion, residential wastes, and mining activities, in addition to the pH/Eh conditions, adsorption, and surface complexation during the chemical weathering are the main factors influence the trace elements distribution in groundwater. Results from this study for the six different groundwater aquifers are a unique insight into the sources and mobility of the observed trace elements in the groundwater and can be used in the assessment of contamination for drinking water wells. The association of trace elements from different aquifers might be useful in tracers studies of water-rock interaction. Due to the enrichment of trace elements in nearshore and crystalline groundwater aquifers in the study area and in similar areas worldwide, treatment technologies, and sustainable water management processes should be applied to prevent severe risks to the communities.






Similar content being viewed by others
References
Abdalla, O., & Al-Abri, R. Y. (2014). Factors affecting groundwater chemistry in regional arid basins of variable lithology: example of Wadi Umairy, Oman. Arabian Journal of Geosciences, 7, 2861–2870.
Abdel Moneim, A. A. (2005). Overview of the geomorphological and hydrogeological characteristics of the Eastern Desert of Egypt. Hydrogeology J, 13, 416–425.
Aiuppa, A., Brusca, L., D’Alessandro, W., Giammanco, S., & Parello, F. (2002). A case study of gas-water-rock interaction in a volcanic aquifer: the south-western flank of Mt. Etna (Sicily). In I. Stober & K. Butcher (Eds.), Water-Rock Interaction in Hydrogeology (pp. 125–145). Netherlands: Kluwert Academic.
Alvarez, M. P., & Carol, E. (2019). Geochemical occurrence of arsenic, vanadium and fluoride in groundwater of Patagonia, Argentina: sources and mobilization processes. Journal of South American Earth Sciences, 89, 1–9.
American Public Health Association (APHA). (2012). Standard methods for the examination of water and wastewater (22th ed.). Washington DC: APHA, American Water Works Association 1496 p.
Angino, E. E., Billings, G. K., & Andersen, N. (1969). Observed variations in the strontium concentration of sea water. Chemical Geology, 1, 145–153.
Anthony, J. W., Bideau, R. A., Bladh, K. W., & Nichols, M. C. (1990). Handbook of mineralogy: volume I: elements, sulfides, sulfosalts. 588 p. Tucson: Mineral Data Publishing.
Armstrong, C. S., Banerjee, S., & Corona, C. (2013). Factor-loading uncertainty and expected returns. Review of Financial Studies, 26, 158–207.
Asran, A. M., & Kabesh, M. (2012). Evolution and geochemical studies on a stromaticmigmatite-amphibolite association in Hafafit area, Central Eastern Desert, Egypt. Journal of Biology and Earth Sciences, 2(1), E17–E33.
Ayedun, H., Gbadebo, A. M., Idowu, O. A., & Arowolo, T. A. (2015). Toxic elements in groundwater of Lagos and Ogun States, Southwest, Nigeria and their human health risk assessment. Environmental Monitoring and Assessment, 187, 351.
Ayres M, Ayres M Jr, Ayres DL, Santos AS (2005) BioEstat 4.0: Aplicações Estatística nas Áreas das Ciências Bio-Médicas. Maringá: Gráfica Ltda.
Bache, B. W. (1986). Aluminium mobilization in soils and waters. Journal of the Geological Society of London, 143, 699–706.
Bakyayita, G. K., Norrström, A. C., & Kulabako, R. N. (2019). Assessment of levels, speciation, and toxicity of trace metal contaminants in selected shallow groundwater sources, surface runoff, wastewater, and surface water from designated streams in Lake Victoria Basin, Uganda. J Environ Public Health, 2019, 18p. https://doi.org/10.1155/2019/6734017.
Balan, E., De Villiers, J. P. R., EeckhoutS, G., Glatzel, P., Toplis, M. J., Fritsch, E., Allard, T., Galoisy, L., & Calas, G. (2006). The oxidation state of vanadium in titanomagnetite from layered basic intrusions. Am Mineral, 91(5-6), 953–956. https://doi.org/10.2138/am.2006.2192.
Banning, A., & Benfer, M. (2017). Drinking water uranium and potential health effects in the German Federal State of Bavaria. International Journal of Environmental Research and Public Health, 14(8), 927. https://doi.org/10.3390/ijerph14080927.
Barceloux, D. G. (1999). Vanadium. Journal of Toxicology. Clinical Toxicology, 37(2), 265–278.
Bernhoft, R. A. (2012). Mercury toxicity and treatment: a review of the literature. Journal of Environmental and Public Health, 460508. https://doi.org/10.1155/2012/460508.
Beswick, A. E. (1976). K and Rb relations in basalts and other mantle derived materials. Is phlogopite the key? Geochimica et Cosmochimica Acta, 40(10), 1167–1183. https://doi.org/10.1016/0016-7037(76)90152-6.
Bhowmik, A. K., Alamdar, A., Katsoyiannis, I., Shen, H., Ali, N., Ali, S. M., Bokhari, H., Schäfer, R. B., Musstjab, S. A., & Eqani, A. S. (2015). Mapping human health risks from exposure to trace metal contamination of drinking water sources in Pakistan. Science of the Total Environment, 538, 306–316. https://doi.org/10.1016/j.scitotenv.2015.08.069.
Bouchard, M. F., Sauvé, S., Barbeau, B., Legrand, M., Brodeur, M. E., Bouffard, T., Limoges, E., Bellinger, D. C., & Mergler, D. (2011). Intellectual impairment in schoolage children exposed to manganese from drinking water. Environmental Health Perspectives, 119, 138–143.
Bricker, O. P., & Jones, B. F. (1995). Main factors affecting the composition of natural waters (pp. 1–5). Boca Raton: CRC Press.
Brooks, W. E., & Matos, G. R. (2009). Mercury recycling in the United States in 2000. USGS, 1–26
Buckley, A. N. (1987). The surface oxidation of cobaltite. Australian Journal of Chemistry, 40(2), 231. https://doi.org/10.1071/CH9870231.
Budianta, W., Fahmi, F. L., & Arifudin, W. I. W. (2019). The distribution and mobility of mercury from artisanal gold mining in river sediments and water, Banyumas, Central Java, Indonesia. Environment and Earth Science, 78, 90–97. https://doi.org/10.1007/s12665-019-8108-4.
Buszka, P. M., Fitzpatrick, J., Watson, L. R., & Kay, R. T. (2007). Evaluation of ground-water and boron sources by use of boron stable-isotope ratios, tritium, and selected water-chemistry constituents near Beverly Shores, Northwestern Indiana, 2004. Scientific Investigations Report Series 2007–5166. https://pubs.usgs.gov/sir/2007/5166/pdf/sir2007-5166_web.pdf.
Calderon, R. L. (2000). The epidemiology of chemical contaminants of drinking water. Food and Chemical Toxicology, 38, S13–S20.
Carretero, S., & Kruse, E. (2015). Iron and manganese content in groundwater on the northeastern coast of the Buenos Aires Province, Argentina. Environment and Earth Science, 73, 1983–1995. https://doi.org/10.1007/s12665-014-3546-5.
Cerny, P., Chapman, R., Teertstra, D. K., & Novák, M. (2003). Rubidium- and cesium-dominant micas in granitic pegmatites. American Minerologist, 88, 1832–1835.
Chao, T. C., Maxwell, S. M., & Wong, S. Y. (1991). An outbreak of aflatoxicosis and boric acid poisoning in Malaysia: a clinic opathological study. The Journal of Pathology, 164, 225–233. https://doi.org/10.1002/path.1711640307.
Chung, J. Y., Yu, S. D., & Hong, Y. S. (2014). Environmental source of arsenic exposure. Journal of Preventive Medicine and Public Health, 47(5), 253–257. https://doi.org/10.3961/jpmph.14.036.
Cooper, R. G., & Harrison, A. P. (2009). The exposure to and health effects of antimony. Indian Journal of Occupational and Environmental Medicine, 13(1), 3–10. https://doi.org/10.4103/0019-5278.50716.
Dar, F. A., Ganai, J. A., Ahmed, S., & Satyanarayanan, M. (2017). Groundwater trace element chemistry of the karstified limestone of Andhra Pradesh, India. Environment and Earth Science, 76, 673–619. https://doi.org/10.1007/s12665-017-6972-3.
Dickinson, K. A., & Morrone, J. F. ( 1982). Distribution of uranium and thorium in the lower Tertiary Orca Group and related rocks in part of the Cordova Quadrangle, southern Alaska: U.S. Geological Survey Open-File Report 82–1032, 10 p.
Edmunds, W. M., & Smedley, P. L. (1996). Ground water geochemistry and health: an overview. In: Appleton JD, Fuge R, McCall GJH (Eds), Environ Geochem Health Geol Soc Special Public No. 113, pp. 91–105.
El-Sayed MA (2000) Radioactive and chemical studies on water of some wellsat Central Eastern Desert, Egypt. M.Sc. Thesis Fac Sci Helwan Univ Egypt 143p
El-Sayed, A. F. M. (2006). Tilapia Culture. Oxfordshire: CABI publishing, CABI International Wallingford.
El-Sayed, M. M., Mohamed, F. H., Furnes, H., & Kanisawa, S. (2002). Geochemistry and petrogenesis of the neoproterozoic granitoids in the Central Eastern Desert, Egypt. Chemie der Erde, 62, 317–346.
Elumalai, V., Brindha, K., & Lakshmanan, E. (2017). Human exposure risk assessment due to heavy metals in groundwater by pollution index and multivariate statistical methods: a case study from South Africa. Water, 9, 234. https://doi.org/10.3390/w9040234.
Embaby, A., Razack, M., Lecoz, M., & Porel, G. (2016). Hydrogeochemical assessment of groundwater in the Precambrian rocks, South Eastern Desert, Egypt. Journal of Water Resource and Protection, 8, 293–310.
Emsley, J. (2001). Nature’s building blocks: an “A-Z guide to the elements”. Oxford: Oxford University Press ISBN 0-19-850340-5, 7 and 8, 1–529.
Etim, E. U. (2017). Occurrence and distribution of arsenic, antimony and selenium in shallow groundwater systems of Ibadan Metropolis, Southwestern Nigerian. Journal of Health and Pollution, 7(13), 32–41. https://doi.org/10.5696/2156-9614-7-13.32.
FAO. (2015). Total renewable water resources per capita. Aquastat 2014, in website; http://www.fao.org/nr/water/aquastat/data/query/results.html, visited in 03/08/2019.
Farrag, A. A., Ibrahim, H. A., El-Hussaini, A. H., & Abdel Kader, A. A. (2005). Geophysical and hydrogeological tools for groundwater exploration and evaluation in the area around Idfu-MarsaAlam road Eastern Desert, Egypt. Assiut University Bulletin for Environmental Researches, 8(1), 67–87.
Filella, M., Belzile, N., & Chen, Y. W. (2002). Antimony in the environment: a review focused on natural waters. I. Occurrence. Earth-Science Reviews, 57, 125–176.
Foster, S., & Loucks, D. P. (2006). Non-renewable groundwater resources: a guidebook on socially-sustainable management for water-policy makers. IHP-VI, Series on Groundwater No. 10, UNESCO, Paris.
Foster, S., Kemper, K., Tuinhof, A., Koundouri, P., Nannin, M., & Garduno, H. (2006). Natural groundwater quality hazards: avoiding problems and formulating mitigation strategies (English). GW Mate Briefing Note series; no. 14. Washington, DC: World Bank http://documents.worldbank.org/curated/en/987261468151479709/Natural-groundwater-quality-hazards-avoiding-problems-and-formulating-mitigation-strategies. Accessed 29 Oct 2018.
Frengstad, B., Skrede, A. K. M., Banks, D., Krog, J. R., & Siewers, U. (2000). The chemistry of Norwegian groundwaters: III. The distribution of trace elements in 476 crystalline bedrock groundwaters, as analysed by ICP-MS techniques. Science of the Total Environment, 246(1), 21–40.
Gill, L. W., Babechuk, M. G., Kamber, B. S., McCormack, T., & Murphy, C. (2018). Use of trace and rare earth elements to quantify autogenic and allogenic inputs within a lowland karst network. Applied Geochemistry, 90, 101–114.
Goldberg, S. (1993). Chemistry and mineralogy of boron in soils. In U. C. Gupta (Ed.), Boron and its role in crop production (pp. 3–44). Boca Raton: CRC Press.
Golekar, R. B., Patil, S. N., & Baride, M. V. (2013). Human health risk due to trace element contamination in groundwater from the Anjani and Jhiri river catchment area in northern Maharashtra, India. Earth Sciences Research Journal, 17(1), 17–23.
Gomaa, M. A., Hamouda, A. A., Abdelfattah, M. E., Emara, M. M., & El-Sabbah, M. M. B. (2013). Assessment of hydrogeochemical processes affecting groundwater quality in the area between Safaga and El-Quseir, Eastern Desert, Egypt. Middle-East Journal of Applied Sciences, 3(4), 129–142.
Greenwood, N. N., & Earnshaw, A. (1997). Chemistry of the elements (2nd ed.). Oxford: Butterworth-Heinemann.
Haan, C. T. (1977). Statistical methods in hydrology. Iowa State Univ Press 378p.
Hammond, G. P. (2000). Energy, environment and sustainable development: a UK perspective. Process Safety and Environmental Protection, 78(4), 304–323.
Harman, H. H. (1960). Modern factor analysis. Chicago: University of Chicago Press 290p.
Harraz, H. Z., Hamdy, M. M., & El-Mamoney, M. H. (2012). Multi-element association analysis of stream sediment geochemistry data for predicting gold deposits in Barramiya gold mine, Eastern Desert, Egypt. Journal of African Earth Sciences, 68, 1–14.
Hasenmueller, E. A., & Criss, R. E. (2013). Multiple sources of boron in urban surface waters and groundwaters. Science of the Total Environment, 447, 235–247. https://doi.org/10.1016/j.scitotenv.2013.01.001.
Health Canada. (2018). Strontium in drinking water - guideline technical document for public consultation. https://www.canada.ca/en/health-canada/programs/consultation-strontium-drinking-water/document.html#2.1.
Heier, K. S., & Billings, G. K. (1970). In K. H. Wedepohl (Ed.), Rubidium in handbook of geochemistry (pp. 37-C-1–37-N-1). Berlin and Heidelberg: Springer-Verlag.
Hem, J. D. (1976). Inorganic chemistry of lead in water. In T. G. Lovering (Ed.), Lead in the environment (pp. 5–11). Washington DC: United States Geological Survey.
Hernández García, M. E., & Fernández Ruiz, L. (2002). Presencia de arsénico de origen natural en las aguas subterráneas del acuífero detrítico del Terciario de Madrid Boletín Geológico y Minero, vol. 113(2) Madrid, España.
Holleman, A. F., Wiberg, E., & Wilberg, N. (2007). Lehebuch der Anoranischen Chemie. Berlin: Walter de Gruyter 2149p.
Jiang, Y., Wu, Y., Groves, C., Yuan, D., & Kambesis, P. (2009). Natural and anthropogenic factors affecting the groundwater quality in the Nandong karst underground river system in Yunan, China. Journal of Contaminant Hydrology, 109, 49–61.
Jones, R. L. (1992). Extractable rubidium in surface horizons of Illinois soils. Soil Science Society of America Journal, 56, 1453–1454.
Kendall, C., Caldwell, E. A., & Snyder, D. (2005). Resources on isotopes—periodic table—boron. U.S.: Geological Survey Isotope Tracers Project http://wwwrcamnl.wr.usgs.gov/isoig/period/b_iig.htm.
Khadra, W. M., Stuyfzand, P. J., & van Breukelen, B. M. (2017). Hydrochemical effects of saltwater intrusion in a limestone and dolomitic limestone aquifer in Lebanon. Applied Geochemistry, 79, 36–51.
Khaled, M. A. (1995). Geological and geophysical investigations for groundwater potentialities in El-Quseir – Abu Ghuson area, South of the Eastern Desert, Egypt. M.Sc. Thesis, Geol Dep Fac Sci Cairo Univ, Cairo, Egypt.
Khalifa, I. H., El-Bialy, M. Z., & Hassan, D. M. (2011). Petrologic and geochemical characterization and mineralization of the metavolcanic rocks of the Heib Formation, Kid Metamorphic Complex, Sinai, Egypt. Geoscience Frontiers, 2(3), 385–402.
Khatri, N., & Tyagi, S. (2015). Influences of natural and anthropogenic factors on surface and groundwater quality in rural and urban areas. Front Life Sci, (1), 23–39.
Kim, C. S., Rytuba, J. J., & Brown Jr., G. E. (2004). Geological and anthropogenic factors influencing mercury speciation in mine wastes: an EXAFS spectroscopy study. Applied Geochemistry, 19, 379–393.
Kothny, E. L. (1973). The three-phase equilibrium of mercury in nature. In E. L. Kothny (Ed.), Trace elements in the environment (pp. 48–80). Washington: American Chemical Society.
Krulík, R., Farská, I., & Prokes, J. (1977). Effect of rubidium, lithium and cesium on brain ATPase and protein kinases. Neuropsychobiology, 3(2-3), 129–134.
Kubier, A., & Pichler, T. (2019). Cadmium in groundwater − a synopsis based on a large hydrogeochemical data set. Science of the Total Environment, 689, 831–842.
Kuck, P. H. (2008). Nickel. In: Mineral commodity summaries. Reston, VA, US Geological Survey. Ref Type: Report.
Kurdehlachin, S., Jafari, H., & Bagheri, R. (2018). Geochemistry of groundwater from a rhyolite aquifer, Northwest Iran. Environment and Earth Science, 77, 704–715. https://doi.org/10.1007/s12665-018-7886-4.
Lawrence, F. W., & Upchurch, S. B. (1982). Identification of recharge areas using geochemical factor analysis. Ground Water, 20, 680–687.
Leung, C., & Jiao, J. J. (2006). Heavy metal and trace element distributions in groundwater in natural slopes and highly urbanized spaces in mid-levels area, Hong Kong. Water Research, 40, 753–767.
Levins, I., & Gosk, E. (2008). Trace elements in groundwater as indicators of anthropogenic Impact. Environmental Geology, 55, 285–290.
Leyssens, L., Vinck, B., Van Der Straeten, C., Wuyts, F., & Maes, L. (2017). Cobalt toxicity in humans—a review of the potential sources and systemic health effects. Toxicology, 387, 43–56.
Liao, F., Wang, G., Shi, Z., Huang, X., Xu, F., Xu, Q., & Guo, L. (2018). Distributions, sources, and species of heavy metals/trace elements in shallow groundwater around the Poyang Lake, East China. Expo Health, 10, 211–227. https://doi.org/10.1007/s12403-017-0256-8.
Lu, Y., Zang, X., Yao, H., Zhang, S., Sun, S., & Liu, F. (2018). Assessment of trace metal contamination in groundwater in a highly urbanizing area of Shenfu New District, Northeast China. Frontiers in Earth Science, 12, 569–582. https://doi.org/10.1007/s11707-018-0677-0.
MacDonald, A. M., Fordyce, F. M., Shand, P., & Dochartaigh, B. E. Ó. (2006). Using geological and geochemical information to estimate the potential distribution of trace elements in Scottish groundwater. British Geological Survey, COMMISSIONED REPORT CR/05/238 N, 39p.
Małecki, J. J., Kadzikiewicz-Schoeneich, M., Eckstein, Y., Szostakiewicz-Hołownia, M., & Gruszczyński, T. (2017). Mobility of copper and zinc in near-surface groundwater as a function of the hypergenic zone lithology at the Kampinos National Park (Central Poland). Environment and Earth Science, 76, 276–216. https://doi.org/10.1007/s12665-017-6527-7.
Mapoma, H. W. T., Xie, X., Nyirenda, M. T., Zhang, L., Kaonga, C. C., & Mbewe, R. (2017). Trace elements geochemistry of fractured basement aquifer in southern Malawi: a case of Blantyre rural. Journal of African Earth Sciences, 131, 43–52.
Martyn, C. N., Barker, D. J. P., Osmond, C., Harris, E. C., Edwardson, J. A., & Lacey, R. F. (1989). Geographical relation between Alzheimer’s disease and A1 in drinking water., Lancet, 1, 59–62.
Maurice, A. E., Bakhit, B. R., Basta, F. F., & Khiamy, A. A. (2013). Geochemistry of gabbros and granitoids (M-and I-types) from the Nubian Shield of Egypt: roots of neoproterozoic intra-oceanicisland arc. Precambrian Research, 224, 397–411.
Mayer JE1, Goldman RH (2016) Arsenic and skin cancer in the USA: the current evidence regarding arsenic-contaminated drinking water. Int J ermatol 55(11):e585-e591. https://doi.org/10.1111/ijd.1331.
Mielke, H. W., Adams, J. L., Chaney, R. L., Mielke, P. W., & Ravlkumar, V. C. (1991). The pattern of cadmium in the environment of five Minnesota cities. Environmental Geochemistry and Health, 13, 29–34.
Mitrakas, M., Mantha, Z., Tzollas, N., Stylianou, S., Katsoyiannis, I., & Zouboulis, A. (2018). Removal of antimony species, Sb (III)/Sb(V), from water by using iron coagulants. Water, 10, 1328. https://doi.org/10.3390/w10101328.
Moore, J. G. (1987). Mount Whitney Quadrangle, Inyo and Tulare Counties, California-Anayltic Data. U.S. Geological Survey Bulletin 1760.
Mora, A., Mahlknecht, J., Rosales-Lagarde, L., & Hernández-Antonio, A. (2017). Assessment of major ions and trace elements in groundwater supplied to the Monterrey metropolitan area, Nuevo León, Mexico. Environmental Monitoring and Assessment, 189, 394.
Morelli, G., Rimondi, V., Benvenuti, M., Medas, D., Costagliola, P., & Gasparon, M. (2017). Experimental simulation of arsenic desorption from Quaternary aquifer sediments following sea water intrusion. Applied Geochemistry, 87, 176–187.
Morland, G., Reimann, C., Strand, T., Skarphagen, H., Banks, D., Bjorvatn, K., Hall, G. E. M., & Siewers, U. (1997). The hydrogeochemistry of Norwegian bed rock groundwater selected parameters (pH,F-,Rn, U,Th, B, Na,Ca) in samples from Vestfold and Hordaland, Norway. Norgesgeologiskeunderssøkelse Bulletin, 432, 103–117.
Muehe, E. M., & Kappler, A. (2014). Arsenic mobility and toxicity in South and South-east Asia—a review on biogeochemistry, health and socio-economic effects, remediation and risk predictions. Environmental Chemistry, 11, 483–495.
Neumayr, P., Hoinkes, G., Puhl, J., Mogessie, A., & Khudeir, A. A. (1998). The Meatiq dome (Eastern Desert, Egypt) a Precambrian metamorphic core complex: petrological and geological evidence. Journal of Metamorphic Geology, 16, 259–279.
Nicholson, J. K., Kendall, M. D., & Osborn, D. (1983). Cadmium and mercury nephrotoxicity. Nature, 304, 633–635.
NPI. (2012). National pollutant inventory – nickel and compounds Fact Sheet. In website: Npi.gov.au.
Nuccio, P. M., & Valenza, M. (1979). Determination of metallic iron, nickel and cobalt in meteorites. Rendiconti della Societa Italiana di Mineralogia e Petrologia, 35(1), 355–360.
Omran AFA. (2013). Application of GIS and remote sensing for water resource management in arid area – Wadi Dahab basin, South Eastern Sinai, Egypt (case-study). PhD Thesis Tübingen, Germany, 1-282
Pacheco, F., & Van der Weijden, C. H. (1996). Contributions of water–rock interactions to the composition of groundwater in areas with a sizable anthropogenic input: a case study of the waters of the Fundao area, central Portugal. Water Resources Research, 32, 3553–3570.
Pelig-Ba, K. B. (1998). trace elements in groundwater from some crystalline rocks in the Upper regions of Ghana. Water, Air, and Soil Pollution, 103, 71–89.
Pelig-Ba, K. B., Parker, A., & Price, M. (2004). trace element geochemistry from the Birrimianmeta sediments of the northern region of Ghana. Water, Air, and Soil Pollution, 153, 69–93.
Pennisi, M., Gonfiantini, R., Grassi, S., & Squarci, P. (2006). The utilization of boron and strontium isotopes for the assessment of boron contamination of the Cecina River alluvial aquifer (central-western Tuscany, Italy). Applied Geochemistry, 21, 643–655.
Plum, L. M., Rink, L., & Haase, H. (2010). The essential toxin: impact of zinc on human health. International Journal of Environmental Research and Public Health, 7(4), 1342–1365. https://doi.org/10.3390/ijerph7041342.
Price, R. C., Stewart, R. B., Woodhead, J. D., & Smith, I. E. (1999). Petrogenesis of high-K arc magmas, evidence from Egmont Volcano, North Island, New Zealand. Journal of Petrology, 40, 167–197.
Ramos Ramos, O. E., Cáceres, L. F., Ormachea Muñoz, M. R., Bhattacharya, P., Israel Quino, I., Quintanilla, J., Sracek, O., Thunvik, R., Bundschuh, J., & García, M. E. (2012). Sources and behavior of arsenic and trace elements in groundwater and surface water in the Poopó Lake Basin, Bolivian Altiplano. Environment and Earth Science, 66, 793–807. https://doi.org/10.1007/s12665-011-1288-1.
Rao, M. S., Gopalkrishnan, R., &Venkatesh, B. R. (2001). Medical geology-an emerging field in environmental science .In National symposium on role of earth sciences (pp. 213–222). Integrated and Related Social issues GSI Special. Pub.No. 65(II).
Rasool, A., Farooqi, A., Masood, S., & Hussain, K. (2016). Arsenic in groundwater and its health risk assessment in drinking water of Mailsi, Punjab, Pakistan. Human and Ecological Risk Assessment, 22(1), 187–202.
Redwan, M., & Abdel Moneim, A. A. (2016). Using Na/K ratios to identify the potential impacts of sewage effluent on groundwater quality in Sohag, Egypt. Groundwater Monitoring & Remediation, 36(4), 62–70.
Redwan, M., & Rammlmair, D. (2017). Flood hazard assessment and heavy metal distributions around Um Gheig mine area, Eastern Desert, Egypt. Journal of Geochemical Exploration, 173, 64–75.
Rehman, F., & Cheema, T. (2017). Boron contamination in groundwater at a sewage waste disposal facility near Jeddah, Saudi Arabia. Environment and Earth Science, 76, 218–219. https://doi.org/10.1007/s12665-017-6528-6.
Reimann C, Caritat Pde (1998) Chemical Elements in the Environment. Factsheets for the Geochemist and Environmental Scientist.: ix+398 pp Berlin, Heidelberg, New York, London, Paris, Tokyo, Hong Kong: Springer-Verlag.
Reverdatto, A., Yu, V. V., & Selyatitskiy, D. A. (2008). Carswell, Geochemical distinctions between “crustal” and mantle-derived peridotites /pyroxenites in high/ultrahigh pressure metamorphic complexes. Russian Geology and Geophysics, 49(2), 73–90. https://doi.org/10.1016/j.rgg.2008.01.002.
Riedel, T., & Kübeck, C. (2018). Uranium in groundwater - A synopsis based on a large hydrogeochemical data set. Water Res, 129, 29–38. https://doi.org/10.1016/j.watres.2017.11.001.
RIGW (1988) Hydrogeological map of Egypt. Scale 1, 2000,000. User guide, Ministry of Irrigation, Cairo, Egypt.
Rwiza, M. J., Kim, K.-W., & S-d, K. (2016). Geochemical distribution of trace elements in groundwater from the North Mara large-scale gold mining area of Tanzania. Groundwater Monit Remed, 36(2), 83–93.
Said, R. (1990). The Geology of Egypt. Rotterdam/Brookfield: AA Balkema 734 p.
Saleh, M. F. (1993). Hydrogeological and hydrochemical Studies on Some Localities in South Eastern Desert, Egypt. Ph.D. Thesis, Suez Canal University, Ismailia. (Unpublished).
Salem, I. A., El-Shibiny, N. H., & Abdel Monsef, M. (2016). Mineralogical and geochemical studies on manganese Depositsat Abu Ghusun Area, South Eastern Desert, Egypt. Journal of Geography and Earth Sciences, 4(2), 51–74.
Scott, G. (2012). Crystal chemistry and synthesis of selected borosilicate minerals. MSc Thesis, the Faculty of Graduate Studies. The University of British Columbia.
Selvam, S., Antony Ravindran, A., Venkatramanan, S., & Singaraja, C. (2017). Assessment of heavy metal and bacterial pollution in coastal aquifers from SIPCOT industrial zones, Gulf of Mannar, South Coast of Tamil Nadu, India. Applied Water Science, 7, 897–913. https://doi.org/10.1007/s13201-015-0301-3.
Sharma, P., Bihari, V., Agarwal, S. K., Verma, V., Kesavachandran, C. N., Pangtey, B. S., Mathur, N., Pal, S. K., Srivastava, M., & Goel, S. K. (2012). Groundwater contaminated with hexavalent chromium [Cr (VI)]: a health survey and clinical examination of community inhabitants (Kanpur, India). PLoS ONE, 7, e47877. https://doi.org/10.1371/Jpone.0047877.
Shiraki, K. (1978). Chromium. In K. H. Wedepohl (Ed.), Handbook of geochemistry, Vol. II-3 (pp. 24B–24O). Berlin: Springer-Verlag.
Shotyk, W., Krachler, M., Aeschbach-Hertig, W., Hillier, S., & Zheng, J. (2010). Trace elements in recent groundwater of an artesian flow system and comparison with snow: enrichments, depletions, and chemical evolution of the water. Journal of Environmental Monitoring, 12, 208–217.
Simmons, E. C. (1999). Rubidium: element and geochemistry. In R. W. Fairbridge (Ed.), Marshall CP (pp. 557–558). The encyclopedia of geochemistry: Chapman and Hall.
Simonsson, M., Court, M., Bergholm, J., Lemarchand, D., & Hillier, S. (2016). Mineralogy and biogeochemistry of potassium in the Skogaby experimental forest, southwest Sweden: pools, fluxes and K/Rb ratios in soil and biomass. Biogeochemistry, 131, 77–102. https://doi.org/10.1007/s10533-016-0266-9.
Suh, J. Y. (2004). Hydrogeochemical studies of groundwater from reclaimed land adjacent to Rozelle Bay, Sydney, Australia. Geosciences Journal, 8, 301–312. https://doi.org/10.1007/BF02910249.
Tahoon, M. A. (2011). Hydrogeochemical and environmental study in the area between Marsa Alam and Baranes, Red Sea Coast, Egypt. Ph.D. Thesis, South Valley University, Qena, 215 p (Unpublished).
Tanji, K., & Valoppi, L. (1989). Groundwater contamination by trace elements. Agriculture, Ecosystems and Environment, (26(3–4), 229–274.
Tebbubtt, T. H. Y. (1983). Relationship between natural water quality and health. Paris: UNESCO.
Tingley, J. V., Bonham, H. F. Jr (1986). Precious-metal mineralization in hot springs systems, Nevada-California. Report 41, NBMG Publications, 136p.
Toccalino, P. L., Norman, J. E., & Scott, J. C. (2012). Chemical mixtures in untreated water from public-supply wells in the U.S.—occurrence, composition, and potential toxicity. Science of the Total Environment, 431, 262–270.
Turekian, K. K., & Wedepohl, K. H. (1961). Distribution of the elements in some major units of the Earth's crust. Bulletin Geological Society of America, 72, 175–192.
Turner, R. D. R., Warne, M. S.-J., Dawes, L. A., Vardy, S., & Will, G. D. (2016). Irrigated greywater in an urban sub-division as a potential source of metals to soil, groundwater and surface water. Journal of Environmental Management, 183(3), 806–817.
Ure, A. M., & Berrow, M. L. (1982). The chemical constituents of soils. In H. J. M. Bowen (Ed.), Environmental Chemistry (pp. 94–202). London: R Soc Chem Burlington House.
Wasserman, M., Renfrew, M. R., Green, A. R., Lopez, L., Tan-McGrory, A., Brach, C., & Betancourt, J. R. (2014). Identifying and preventing medical errors inpatients with limited English proficiency: key findings and tools for the field. J Healthc Qual, 36(3), 5–16. https://doi.org/10.1111/jhq.1206.
Wickleder, M. S., Fourest, B., & Dorhout, P. K. (2006). Thorium. In L. R. Morss, N. M. Edelstein, & J. Fuger (Eds.), The Chemistry of the Actinide and Transactinide Elements (pp. 52–160). Springer.
World Health Organization (WHO). (2011). Guidelines for drinking-water quality, fourth edition. ISBN: 9789241548151. 564p.
Wright, M. T., & Belitz, K. (2010). Factors controlling the regional distribution of vanadium in groundwater. Groundwater, 48(4), 515–525. https://doi.org/10.1111/j.1745-6584.2009.00666.x.
Young, R. (1957). The geochemistry of cobalt. Geochimica et Cosmochimica Acta, 13(1), 28–41.
Youssef, L. A. (2011). Hydrochemistry of some wells and stable isotope evaluation, South eastern Desert, Egypt. PhD Banha Univ Fac Sci Geol Dep 303. 104p.
Zaki, S. R., Redwan, M., Masoud, A. M., & Abdel Moneim, A. A. (2019). Chemical characteristics and assessment of groundwater quality in Halayieb area, southeastern part of the Eastern Desert, Egypt. Geosciences Journal, 23, 149–164. https://doi.org/10.1007/s12303-018-0020-5.
Acknowledgments
Anonymous reviewers are acknowledged for the helpful comments on the manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Embaby, A., Redwan, M. Sources and behavior of trace elements in groundwater in the South Eastern Desert, Egypt. Environ Monit Assess 191, 686 (2019). https://doi.org/10.1007/s10661-019-7868-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10661-019-7868-3