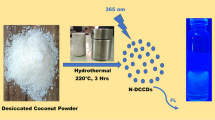
Abstract
A fluorescent composite material fabricated from nitrogen-doped carbon dots with polyvinyl alcohol (PVA)/polyvinylpyrrolidone (PVP)/citric acid (CA) hydrogel was synthesized using a microwave-assisted hydrothermal method. The composite was used as a metal ion sensor and adsorbent to remove chromium (Cr(VI)) from water. The chemical structure and Cr(VI) removal performance of the fluorescent composite films were also characterized. Fluorescent quenching upon Cr(VI) adsorption showed that Cr(VI) binding was attributed to the N-doped carbon dots. The results were confirmed by several analytical techniques, including X-ray photoelectron spectroscopy (XPS), Fourier transform infrared spectroscopy (FTIR) and X-ray absorption spectroscopy (XAS). The mechanism of Cr(VI) removal from water by the fluorescent composite film was based on the adsorption and subsequent reduction of N-doped carbon dots within the 3D porous composite film. XPS measurements showed that 53.2% Cr(III) and 46.8% Cr(VI) were present on the composite surface after Cr(VI) adsorption. Moreover, XAS revealed a change in the oxidation state of Cr(VI) to Cr(III) after adsorption and in the Cr–O bond length (1.686 Å to 2.284 Å) after reduction. The Cr(VI) adsorption capacity of the composite film was 4.90 mg g−1 at pH 4 and fit the pseudo-second-order kinetic and Freundlich models. The results of this study could be used as a platform to further apply CDs/HD composites to remove Cr(VI) from water sources.
Graphical Abstract







Similar content being viewed by others
References
Abd El-Mohdy, H. L., & Ghanem, S. (2009). Biodegradability, antimicrobial activity and properties of PVA/PVP hydrogels prepared by γ-irradiation. Journal of Polymer Research, 16, 1–10. https://doi.org/10.1007/s10965-008-9196-0
Appel, E. A., Barrio, J., Loh, X. J., & Scherman, O. A. (2012). Supramolecular polymeric hydrogels, Supramolecular polymeric hydrogels. Chemical Society Reviews, 41, 6195–6214. https://doi.org/10.1039/C2CS35264H
Barman, M. K., Jana, B., Bhattacharyya, S., & Patra, A. (2014). Photophysical properties of doped carbon dots (N, P, and B) and their influence on electron/hole transfer in carbon dots–nickel(II) phthalocyanine conjugates. Journal of Physical Chemistry C, 118, 20034–20041. https://doi.org/10.1021/jp507080c
Buddu, V. M., Abburi, K., Talbott, J. L., & Smith, E. D. (2003). Removal of hexavalent chromium from wastewater using a new composite chitosan biosorbent. Environmental Science & Technology, 37, 4449–4456. https://doi.org/10.1021/es021013a
Chen, Y., An, D., Sun, S., Gao, J., & Qian, L. (2018). Reduction and removal of chromium VI in water by powered activated carbon. Materials, 11, 269. https://doi.org/10.3390/ma11020269
Ding, H., Li, X., Chen, X., Wei, J., Li, X., & Xiong, H. (2020). Surface states of carbon dots and their influences on luminescence. Journal of Applied Physics, 127, 231101. https://doi.org/10.1063/1.5143819
Dong, X., Ma, L. Q., & Li, Y. (2021). Characteristics and mechanisms of hexavalent chromium removal by bio char from sugar beet tailing. Journal of Hazardous Materials, 190, 909–915. https://doi.org/10.1016/j.jhazmat.2011.04.008
Gaur, A., & Shrivastava, B. D. (2012). A comparative study of the methods of speciation using X-ray absorption fine structure. Acta Physica Polonica A, 121(3), 647–652. https://doi.org/10.12693/APhysPolA.121.647
Gayen, B., Palchoudhury, S., & Chowdhury, J. (2019). Carbon dots: A mystic star in the world of nanoscience. Journal of Nanomaterials. https://doi.org/10.1155/2019/3451307
Godiya, C. B., Cheng, X., Li, D., Chen, Z., & Lu, X. (2019). Carboxymethyl cellulose/polyacryamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. Journal of Hazardous Materials, 364, 28–38. https://doi.org/10.1016/j.jhazmat.2018.09.076
Gogoi, N., Barooah, M., Maajumdar, G., & Chowdhury, D. (2015). Carbon dots rooted agarose hydrogel hybrid platform for optical detection and separation of heavy metal ions. ACS Applied Materials & Interfaces, 7, 3058. https://doi.org/10.1021/am506558d
Gorzin, F., & Abadi, M. M. B. R. (2018). Adsorption of Cr(VI) from aqueous solution by adsorbent prepared from paper mill sludge: Kinetics and thermodynamics studies. Adsorption Science & Technology, 36, 149–169. https://doi.org/10.1177/0263617416686976
Hao, D., & Liang, Y. (2020). Adsorption of Cu2+, Cd2+ and Pb2+ in wastewater by modified chitosan hydrogel. Environmental Technology, 43, 876–884. https://doi.org/10.1080/09593330.2020.1807612
Ho, Y. S., & McKay, G. (1999). Pseudo-second order model for sorption processes. Process Biochemistry, 34, 451–465. https://doi.org/10.1016/S0032-9592(98)00112-5
Hu, M., Wei, Y., Yang, Y., & Lee, J. (2004). X-ray absorption spectroscopy study of chromium recovered from Cr(VI)-containing water with rice husk. Journal of Physics: Condensed Matter., 16, S3473–S3478. https://doi.org/10.1088/0953-8984/16/33/007
Husain, M. S. B., Gupta, A., Alashwal, B. Y., & Sharma, S. (2018). Synthesis of PVA/PVP based hydrogel for biomedical applications: A review. Energy Sources, Part a: Recovery, Utilization, and Environmental Effects, 40, 2388–2393. https://doi.org/10.1080/15567036.2018.1495786
Jiang, C., Wang, X., Wang, G., Hao, C., Li, X., & Li, T. (2019). Adsorption performance of a polysaccharide composite hydrogel based on crosslinked glucan/chitosan for heavy metal ions. Composites Part b: Engineering., 169, 45–54. https://doi.org/10.1016/j.compositesb.2019.03.082
Jiang, Y., Wang, Y., Meng, F., Wang, B., Chong, Y., & Zhu, C. (2015). N-doped carbon dots synthesized by rapid microwave irradiation as highly fluorescent probes for Pb2+. New Journal of Chemistry, 39, 3357–3360. https://doi.org/10.1039/C5NJ00170F
Jlassi, K., Eid, K., Sliem, M. H., Abdullah, A. M., Chehimi, M. M., & Krupa, I. R. (2020). Rational synthesis I characterization, and application of environmentally friendly (polymer-carbon dot) hybrid composite film for fast and efficient UV-assisted Cd2+ removal from water. Environmental Sciences Europe, 32, 1–13. https://doi.org/10.1186/s12302-020-0292-z
Kahrizi, P., Mohseni-Shahri, F. S., & Moeinpour, F. (2018). Adsorption removal of cadmium from aqueous solutions using NiFe2O4/hydroxyapatite/graphene quantum dots as a novel nano-adsorbent. Journal of Nanostructures in Chemistry., 8, 441–452. https://doi.org/10.1007/s40097-018-0284-3
Li, J., Xu, Z., Wu, W., Jing, Y., Dai, H., & Fang, G. (2018). Nanocellulose/Poly(2-(dimethylamino)ethylmethacrylate) Interpenetrating polymer network hydrogels for removal of Pb(II) and Cu(II) ions. Colloids Surface A, 538, 474–480. https://doi.org/10.1016/j.colsurfa.2017.11.019
Li, W., Wang, S., Li, Y., Ma, C., Huang, Z., Wang, C., Li, J., Chen, Z., & Liu, S. (2017). One-step hydrothermal synthesis of fluorescent nano crystalline cellulose/carbon dot hydrogels. Carbohydrate Polymers, 175, 7–17. https://doi.org/10.1016/j.carbpol.2017.07.062
Liu, P., Ptacek, C. J., Blowes, D. W., Finfrock, Y. Z., & Liu, Y. (2020). Characterization of chromium species and distribution during Cr(VI) removal by biochar using confocal micro-X-ray fluorescence redox mapping and X-ray absorption spectroscopy. Environment International, 134, 105216. https://doi.org/10.1016/j.envint.2019.105216
Liu, P., Ptacek, C. J., Blowes, D. W., Finfrock, Y. Z., Steinepreis, M., & Budimir, F. (2019). A method for redox mapping by confocal micro-X-ray fluorescence imaging: Using chromium species in a biochar particle as an example. Analytical Chemistry, 91, 5142–5149. https://doi.org/10.1021/acs.analchem.8b05718
Lu, K., Lin, J., Lin, C., Chen, C., & Yeh, Y. (2019). A fluorometric paper test for chromium(VI) based on the use of N-doped carbon dots. Microchimica Acta., 186, 227. https://doi.org/10.1007/s00604-019-3337-5
Luo, Q., Huang, X., Luo, Y., Yuan, H., Ren, T., Li, X., Xu, D., Guo, X., & Wu, Y. (2021a). Fluorescent chitosan-based hydrogel incorporating titanate and cellulose nanofibers modified with carbon dots for adsorption and detection of Cr(VI). Chemical Engineering Journal, 407, 127050. https://doi.org/10.1016/j.cej.2020.127050
Luo, Q., Yuan, H., Zhang, M., Jiang, P., Liu, M., Xu, D., Guo, X., & Wu, Y. A. (2021b). A 3D porous fluorescent hydrogel based on amino-modified carbon dots with excellent sorption and sensing abilities for environmentally hazardous Cr(VI). Journal of Hazardous Materials, 401, 123432. https://doi.org/10.1016/j.jhazmat.2020.123432
Miretzky, P., & Fernandez Cirelli, A. (2010). Cr(VI) and Cr(III) removal from aqueous solution by raw and modified lignocellulose materials: A review. Journal of Hazardous Materials, 180, 1–19. https://doi.org/10.1016/j.jhazmat.2010.04.060
Muhammad, A., Shah, A. H. A., & Bilal, S. (2020). Effective adsorption of hexavalent chromium and divalent nickel ions from water through polyaniline, iron oxide, and their composites. Applied Science, 10, 2882. https://doi.org/10.3390/app10082882
Neal, A., & Little, B. (2002). Oxidation state of chromium associated with cell surfaces of Shewanella oneidensis during chromate reduction. Applied Surface Science, 202, 150–159. https://doi.org/10.1016/S0169-4332(02)00550-0
Ozkaya, B. (2005). Adsorption and desorption of phenol on activated carbon and a comparison of isotherm models. Journal of Hazardous Materials., 129, 158–163. https://doi.org/10.1016/j.jhazmat.2005.08.025
Park, D., Yun, Y., & Park, J. M. (2008). XAS and XPS studies on chromium-binding groups of biomaterial during Cr(VI) biosorption. Journal of Colloid and Interface Science, 317, 54–61. https://doi.org/10.1016/j.jcis.2007.09.049
Rajapaksha, A. U., Alam, Md. S., Chen, N., Alessi, D. S., Igalavithana, A. D., Tsang, D. C. W., & Ok, Y. S. (2018). Removal of hexavalent chromium in aqueous solutions using biochar: Chemical and spectroscopic investigations. Science of the Total Environment, 625, 1567–1573. https://doi.org/10.1016/j.scitotenv.2017.12.195
Ravel, B., & Neville, M. (2005). ATHENA, ARTEMIS, HEPHAESTUS: Data analysis for X-ray absorption spectroscopy using IFEFFIT. Journal of Synchrotron Radiation, 12, 537–541. https://doi.org/10.1107/S0909049505012719
Ren, G., Hou, X., Kang, Y., Zhang, R., Zhang, M., Liu, W., Li, L., Wei, S., Wang, H., Wang, B., & Diao, H. (2020). Effective preparation of nitrogen-doped fluorescent carbon dots for highly sensitive detection of metronidazole and live cell imaging. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 234, 118251. https://doi.org/10.1016/j.saa.2020.118251
Ren, J., Achilles, D. S., Golnak, R., Yuzawa, H., Xiang, J., Nagasaki, M., Reisner, E., & Petit, T. (2019). Uncovering charge transfer between carbon dots and water by in situ soft X-ray absorption spectroscopy. Journal of Physical Letters., 10, 3843–3848. https://doi.org/10.1021/acs.jpclett.9b01800
Sanchez-Hachair, A., & Hofmann, A. (2018). Hexavalent chromium quantification in solution: Comparing direct UV-visible spectrometry with 1,5-diphenylcarbazide colorimetry. Comptes Rendus Chimie, 21, 890–896. https://doi.org/10.1016/j.crci.2018.05.002
Satyanarayana, J., Murthy, G. S., & Sasidhar, P. (1999). Adsorption studies of cesium on zirconium molybdoarsenate. Waste Management, 19, 427–432. https://doi.org/10.1016/S0956-053X(99)00200-7
Shi, Y., Xiong, D., Liu, Y., Wang, N., & Zhao, X. (2016). Swelling, mechanical and friction properties of PVA/PVP hydrogels after swelling in osmotic pressure solution. Material Science and Engineering: C, 65, 172–180. https://doi.org/10.1016/j.msec.2016.04.042
Singh, B., & Pal, L. (2011). Radiation crosslinking polymerization of sterculia polysaccharide-PVA-PVP for making hydrogel wound dressings. International Journal of Biological Macromolecules, 48, 501–510. https://doi.org/10.1016/j.ijbiomac.2011.01.013
Singh, S., Shauloff, N., & Jelinek, R. (2019). Solar-Enabled water remediation via recyclable carbon dot/hydrogel composites. ACS Sustainable Chemistry & Engineering, 7, 13186–13194. https://doi.org/10.1021/acssuschemeng.9b02342
So, R. C., Sanggo, J. E., Jin, L., Diaz, J. M. A., Guerrero, R. A., & He, J. (2017). Gram-scale synthesis and kinetic study of bright carbon dots from citric acid and citrus japonica via a microwave-assisted method. ACS Omega, 2, 5196–5208. https://doi.org/10.1021/acsomega.7b00551
Son, D., Cho, S., Nam, J., Lee, H., & Kim, M. (2020). X-ray-Based spectroscopy techniques for characterization of polymer nanocomposite materials at a molecular level. Polymers, 12, 1053. https://doi.org/10.3390/polym12051053
Song, S., Liang, F., Li, M., Du, F., Dong, W., Gong, X., Shuang, S., & Dong, C. (2019). A label-free nano-probe for sequential and quantitative determination of Cr(VI) and ascorbic acid in real samples based on S and N dual-doped carbon dots. Spectrochimica Acta Part a: Molecular and Biomolecular Spectroscopy, 215, 58–68. https://doi.org/10.1016/j.saa.2019.02.065
Song, Z., Chen, X., Gong, X., Gao, X., Dia, Q., Nguyen, T. T., & Guo, M. (2020). Luminescent carbon quantum dots/nanofibrillated cellulose composite aerogel for monitoring adsorption of heavy metal ions in water. Optical Materials, 100, 109642. https://doi.org/10.1016/j.optmat.2019.109642
Sui, B., Li, Y., & Yang, B. (2020). Nanocomposite hydrogels based on carbon dots and polymers. Chinese Chemical Letters, 31, 1443–1447. https://doi.org/10.1016/j.cclet.2019.08.023
Takara, E. A., Pereira, S. V., Scala-Benuzzi, M. L., Fernandez-Baldo, M. A., Raba, J., & Messina, G. A. (2019). Novel electrochemical sensing platform based on a nanocomposite of PVA/PVP/RGO applied to IgG anti- Toxoplasma gondii anti bodies quantitation. Talanta, 195, 699–705. https://doi.org/10.1016/j.talanta.2018.11.070
Thongsuksaengcharoen, S., Samosorn, S., & Songsrirote, K. (2020). A facile synthesis of self-catalytic hydrogel films and their application as a wound dressing material coupled with natural active compounds. ACS Omega, 5, 25973–25983. https://doi.org/10.1021/acsomega.0c03414
Tran, V. V., Park, D., & Lee, Y. (2018). Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environmental Science Pollution Research, 25, 24569–24599. https://doi.org/10.1007/s11356-018-2605-y
Valadi, F. M., Shahsavari, S., Akbarzadeh, E., & Gholami, M. R. (2022). Preparation of new MOF-808/chitosan composite for Cr(VI) adsorption from aqueous solution: Experimental and DFT study. Carbohydrate Polymers, 288, 119383. https://doi.org/10.1016/j.carbpol.2022.119383
Valizadeh, B., Nguyen, T. N., Kampouri, S., Sun, D. T., Mensi, M. D., Stylianou, K., Smit, B., & Queen, W. L. (2020). A novel integrated Cr(VI) adsorption-photoreduction system using MOF@polymer composite beads. Journal of Materials Chemistry A, 8, 9629–9637. https://doi.org/10.1039/D0TA01046D
Wang, C. J., Liu, X., Milcovich, G., Chen, T., Durack, E., Mallen, S., Ruan, Y., Weng, X., & Hudson, S. P. (2018). Co-reductive fabrication of carbon nanodots with high quantum yield for bioimaging of bacteria. Beilstein Journal of Nanotechnology, 9, 137–145. https://doi.org/10.3762/bjnano.9.16
Wang, Z., Long, P., Feng, Y., Qin, C., & Feng, W. (2017). Surface passivation of carbon dots with ethylene glycol and their high-sensitive to Fe3+. RSC Advances, 7, 2810. https://doi.org/10.1039/C6RA25465A
WHO Guidelines for drinking water quality, (1997). Fourth ed. http://www.apps.who.int (Accessed 10 September 2022).
Xia, S., Song, Z., Jeyakumar, P., Shaheen, S. M., Rinklebe, J., & Ok, Y. S. (2019). A critical review on bioremediation technologies for Cr(VI)-contaminated soils and wastewater. Critical Reviews in Environmental Science and Technology, 49, 1027. https://doi.org/10.1080/10643389.2018.1564526
Yuan, H., Peng, J., Ren, T., Luo, Q., Luo, Y., Zhang, N., Huang, Y., Guo, X., & Wu, Y. (2021). Novel fluorescent lignin-based hydrogel with cellulose nanofibers and carbon dots for highly efficient adsorption and detection of Cr(VI). Science of the Total Environment, 760, 143395. https://doi.org/10.1016/j.scitotenv.2020.143395
Yuan, N., Majeed, M. H., Bajnoczi, E. G., Persson, A. R., Wallenberg, L. R., Inge, A. K., Heidenreich, N., Stock, N., Zou, X., Wendt, O. F., & Persson, I. (2019). In situ XAS study of the local structure and oxidation state evolution of palladium in a reduced graphene oxide supported Pd(II) carbene complex during an undirected C-H acetoxylation reaction. Catalysis Science & Technology, 9, 2025–2031. https://doi.org/10.1039/C8CY02430H
Zhang, R., & Chen, W. (2014). Nitrogen-doped carbon quantum dots: Facile synthesis and application as a “turn-off” fluorescent probe for detection of Hg2+ ions. Bioelectronics, 55, 83–90. https://doi.org/10.1016/j.bios.2013.11.074
Zheng, M., Xie, Z., Qu, D., Li, D., Du, P., Jiang, X., & Sun, Z. (2013). On-off-on fluorescent carbon dots nano sensor for recognition of chromium(VI) and ascorbic acid based ion the inner filter effect. ACS Applied Materials & Interfaces, 5, 13242–13247. https://doi.org/10.1021/am4042355
Zhou, Y., Zhang, L., Fu, S., Zheng, L., & Zhan, H. (2012). Adsorption behavior of Cd2+, Pb2+, and Ni2+ from aqueous solutions on cellulose-based hydrogels. BioResources, 7, 2752–2765.
Acknowledgements
The authors thank for SLRI for the allocation of synchrotron radiation beamtime. The authors would like to thank Dr. Penphitcha Amonpattaratkit for assistant in XAS conceptual discussions. Assistance provided by beamline 8 (BL8) staff members of the synchrotron facility is also gratefully acknowledged.
Funding
This work was supported by the Faculty of Science, Srinakharinwirot University, Thailand [grant numbers 123/2564, 124/2564, 125/2564].
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by WB, TP, JC and PP. The first draft of the manuscript was written by PP, WB and KS, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Busayaporn, W., Songsrirote, K., Phlialamkheak, T. et al. Synthesis and application of fluorescent N-doped carbon dots/hydrogel composite for Cr(VI) adsorption: Uncovering the ion species transformation and fluorescent quenching mechanism. Environ Geochem Health 45, 5293–5309 (2023). https://doi.org/10.1007/s10653-023-01576-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10653-023-01576-x