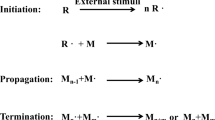
Abstract
During the last decade, hydrogels have been used as potential adsorbents for removal of contaminants from aqueous solution. To improve the adsorption efficiency, there are numerous different particles that can be chosen to encapsulate into hydrogels and each particle has their respective advantages. Depending on the type of pollutants and approaching method, the particles will be used to prepare hydrogels. The hydrogels commonly applied in water/wastewater treatment was mainly classified into three classes according to their shape included hydrogel beads, hydrogel films, and hydrogel nanocomposites. In review of many recently research papers, we take a closer look at hydrogels and their applications for removal of contaminants, such as heavy metal ion, dyes, and radionuclides from water/wastewater in order to elucidate the reactions between contaminants and particles and potential for recycling and regeneration of the post-treatment hydrogels.

ᅟ















Similar content being viewed by others
References
Abachi MQA, Awady NSA, Al-Anbakey AM (2013) Evaluation of poly acrylic acid(PAA) hydrogel beads as adsorbent for the removal of lead(II) ion from water. J Al-Nahrain Univ 16:30–39
Abdelwahab HE, Hassan SY, Mostafa MA, Sadek MME (2016) Synthesis and characterization of glutamic-chitosan hydrogel for copper and nickel removal from wastewater. Molecules 21:14
Agrawal A, Pandey RS, Sharma B (2010) Water pollution with special reference to pesticide contamination in India. J Water Resource Prot 2:432–448
Ahmed EM (2015) Hydrogel: preparation, characterization, and applications. a review J Adv Res 6:105–121
Akpomie KG, Dawodu FA (2015) Potential of a low-cost bentonite for heavy metal abstraction from binary component system. Beni-Suef Uni J Appl Sci 4:1–13
Akpomie KG, Dawodu FA (2016) Acid-modified montmorillonite for sorption of heavy metals from automobile effluent. Beni-Seuf Univ J Appl Sci 5:1–12
Al-Mubaddel FS, Aijaz MO, Haider S, Haider A, Almasry WA, Al-Fatesh AS (2015) Synthesis of chitosan based semi-IPN hydrogels using epichlorohydrine as crosslinker to study the adsorption kinetics of rhodamine B. Desalin Water Treat 57:17523–17536
Ali ZA, Venkatesan J, Kim SK, Sudha PN (2011) Beneficial effect of chitosan-g-polyacrylamide copolymer in removal of heavy metals from industrial dye effluents. Inter J Environ Sci 1:820–833
Antić KM, Babić MM, Vuković JJJ, Vasiljević-Radović DG, Onjia AE, Filipović JM, Tomić SL (2015) Preparation and characterization of novel P(HEA/IA) hydrogels for Cd2+ ion removal from aqueous solution. Appl Surf Sci 338:178–189
Antić KM, Babić MM, Vuković JS, Onjia AE, Filipović JM, Tomić SL (2016) Removal of Pb2+ from aqueous solution by P(HEA/IA) hydrogels. Hem Ind 70:695–705
Azbar N, Yonar T, Kestioglu K (2004) Comparison of various advanced oxidation processes and chemical treatment methods for COD and color removal from a polyester and acetate fiber dyeing effluent. Chemosphere 55:35–43
Babel S, Kurniawan TA (2003) Low-cost adsorbents for heavy metals uptake from contaminated water: a review. J Hazard Mater 97:219–243
Babu VR, Sairam M, Hosamani KM, Aminabhavi TM (2007) Preparation of sodium alginate–methylcellulose blend microspheres for controlled release of nifedipine. Carbohydr Polym 69:241–250
Baruah U, Konwar A, Chowdhury D (2016) A sulphonated carbon dot–chitosan hybrid hydrogel nanocomposite as an efficiention-exchange film for Ca2+ and Mg2+ removal. Nanoscale 8:8542–8546
Bhattacharyya R, Ray SK (2013) Kinetic and equilibrium modeling for adsorption of textile dyes in aqueous solutions by carboxymethyl cellulose/poly(acrylamide-co-hydroxyethyl methacrylate) semi-interpenetrating network hydrogel. Polym Eng Sci 2013:2439–2453
Bitinis N, Hernandez M, Verdejo R, Kenny JM, Lopez-Manchado MA (2011) Recent advances in clay/polymer nanocomposites. Adv Mater 23:5229–5236
Blackburn RS (2004) Natural polysaccharides and their interactions with dye molecules: applications in effluent treatment. Environ Sci Technol 38:4905–4909
Cai J, Zhang L, Zhou J, Li H, Chen H, Jin H (2004) Novel fibers prepared from cellulose in NaOH/urea aqueous solution. Macromol Rapid Commun 25:1558–1562
Carlmark A, Malmström E, Malkoch M (2013) Dendritic architectures based on bis-MPA: functional polymeric scaffolds for application-driven research. Chem Soc Rev 42:5858–5879
Chai L, Wang J, Wang H, Zhang L, Yud W, Mai L (2015) Porous carbonized graphene-embedded fungus film as an interlayer for superior Li–S batteries. Nano Energy 17:224–232
Chatterjee S, Chatterjee S, Chatterjee BP, Das AR, Guha AK (2005) Removal of eosin Y from aqueous solution by chitosan hydro beads. J Colloid Interface Sci 288:30–35
Chatterjee S, Chatterjee S, Chatterjee BP, Guha AK (2007) Adsorptive removal of Congo red, a carcinogenic textile dye by chitosan hydrobeads: binding mechanism, equilibrium and kinetics. Colloids Surf A Physicochem Eng Asp 299:146–152
Chatterjee S, HanWoo S (2009) The removal of nitrate from aqueous solutions by chitosan hydrogel beads. J Hazard Mater 164:1012–1018
Chatterjee S, Lee DS, Lee MW, Woo SH (2009) Enhanced adsorption of Congo red from aqueous solutions by chitosan hydrogel beads impregnated with cetyl trimethyl ammonium bromide. Bioresour Technol 100:2803–2809
Chauhan GS, Kumar S, Kumari A, Sharma R (2003) Study on the synthesis, characterization, and sorption of some metal ions on gelatin- and acrylamide-based hydrogels. J Appl Polym Sci 90:3856–3871
Chen S, Yue Q, Gao B, Xu X (2010) Equilibrium and kinetic adsorption study of the adsorptive removal of Cr(VI) using modified wheat residue. J Colloid Interface Sci 349:256–264
Chen X, Zhou S, Zhang L, You T, Xu F (2016) Adsorption of heavy metals by graphene oxide/cellulose hydrogel prepared from NaOH/urea aqueous solution. Materials 9:582–596
Chien SH, Clayton WR (1980) Application of Elovich equation to the kinetics of phosphate release and sorption in soils. Soil Sci Soc Am J 44:265–168
Crini G (2006) Non-conventional low-cost adsorbents for dye removal: a review. Bioresour Technol 97:1061–1085
Cui W, Ji J, Cai Y-F, Li H, Ran R (2015) Robust, anti-fatigue, and self-healing graphene oxide/hydrophobically associated composite hydrogels and their use as recyclable adsorbents for dye wastewater treatment. J Mater Chem A 3:17445–17458
Demiral H, Güngor C (2016) Adsorption of copper(II) from aqueous solutions on activated carbon prepared from grape bagasse. J Clean Prod 124:103–113
Deng S, Bai R, Chen JP (2003) Aminated polyacrylonitrile fibers for lead and copper removal. Langmuir 19:5058–5064
Du M, Guo B, Jia D (2010) Newly emerging applications of halloysite nanotubes: a review. Polym Int 59:574–582
Dwivedi C, Gupta A, Chaudhary A, Nandi CK (2014) Gold nanoparticle chitosan composite hydrogel beads show efficient removal of methyl parathion from waste water. RSC Adv 4:39830–39838
Dwivedi C, Pathak SK, Kumar M, Tripathib SC, Bajaja PN (2015) Preparation and characterization of potassium nickel hexacyanoferrate-loaded hydrogel beads for the removal of cesium ions. Environ Sci: Water Res Technol 1:153–160
Fekete T, Borsa J, Takács E, Wojnárovits L (2017) Synthesis of carboxymethylcellulose/starch superabsorbent hydrogels by gamma-irradiation. Chem Cent J 11:1–10
Flory PJ (1953). Principles of polymer chemistry. Cornell University: Ithaca, New York 1953
Foo KY, Hameed BH (2010) Insights into the modeling of adsorption isotherm systems. Chem Engin J 156:2–10
Freundlich HMF (1906) Over the adsorption in solution. J Phys Chem 57:385–471
Fritz JS (2005) Factors affecting selectivity in ion chromatography. J Chromatogr A 1085:8–17
Fu Y, Viraraghavan T (2001) Fungal decolorization of dye waste waters: a review. Bioresour Technol 79:251–262
Gao H, Sun Y, Zhou J, Xu R, Duan H (2013) Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification. ACS Appl Mater Interfaces 5:425–432
Gercel O, Gercel HF (2007) Adsorption of lead(II) ions from aqueous solutions by activated carbon prepared from biomass plant material of Euphorbia rigida. Chem. Engin. J. 132:289–297
Gogoi N, Barooah M, Majumdar G, Chowsdury D (2015) Carbon dots rooted agarose hydrogel hybrid platform for optical detection and separation of heavy metal ions. ACS Appl Mater Interfaces 7:3058–3067
Golmohamadi M, Davis TA, Wilkinson KJ (2012) Diffusion and partitioning of cations in an agarose hydrogel. J Phys Chem A 116:6506–6510
Gong Z, Zhang G, Zeng X, Li J, Li G, Huang W, Sun R, Wong C (2016) High-strength, tough, fatigue resistant, and self-healing hydrogel based on dual physically cross-linked network. ACS Appl Mater Interfaces 8:24030–24037
Guilherme MR, Reis AV, Paulino AT, Moia TA, Mattoso LHC, Tambourgi EB (2010) Pectin-based polymer hydrogel as a carrier for release of agricultural nutrients and removal of heavy metals from wastewater. J Appl Polym Sci 117:3146–3154
Guo H, Jiao T, Zhang Q, Guo W, Peng Q, Yan X (2015) Preparation of graphene oxide-based hydrogels as efficient dye adsorbents for wastewater treatment. Nanoscale Res Lett 10:272
Gurses A, Dogar C, Yalcın M, Acıkyıldız M, Bayrak R, Karaca S (2006) The adsorption kinetics of the cationic dye, methylene blue, onto clay. J Hazard Mater B 131:217–228
Han R, Ding D, Xu Y, Zou W, Wang Y, Li Y, Zou L (2008) Use of rice husk for the adsorption of Congo red from aqueous solution in column mode. Bioresour Technol 99:2938–2946
Haraguchi K, Takehisa T, Fan S (2002) Effects of clay content on the properties of nanocomposite hydrogels composed of poly(n-isopropylacrylamide) and clay. Macromolecules 35:10162–10171
Haraguchi K, Farnworth R, Ohbayashi A, Takehisa T (2003) Compositional effects on mechanical properties of nanocomposite hydrogels composed of poly(n,n-dimethylacrylamide) and clay. Macromolecules 36:5732–5741
Haubner K, Murawski J, Olk P, Eng LM, Ziegler C, Adolphi B, Jaehne E (2010) The route to functional graphene oxide. ChemPhysChem 11:2131–2139
Heydari A, Sheibani H (2015) Fabrication of poly(b-cyclodextrin-co-citric acid)/bentonite clay nanocomposite hydrogel: thermal and absorption properties. RSC Adv 5:2438–82449
Ho YS, McKay G (1999) Pseudo-second order model for sorption processes. Process Biochem 34:451–465
Hong S, Wen C, He J, Gan F, Ho Y-S (2009) Adsorption thermodynamics of methylene blue onto bentonite. J Hazard Mater 167:630–633
Howard BJ, Beresford NA, Hove K (1991) Transfer of radiocesium to ruminants in natural and semi-natural ecosystems and appropriate countermeasures. Health Phys 61:715–725
Hwang D-C, Damodaran S (1997) Metal-chelating properties and biodegradability of an ethylenediaminetetraacetic acid dianhydride modified soy protein hydrogel. J Appl Polym Sci 64:891–901
Inal M, Erduran N (2015) Removal of various anionic dyes using sodium alginate/poly(N-vinyl-2-pyrrolidone) blend hydrogel beads. Polym Bull 72:1735–1752
Jana S, Pradhan SS, Tripathy T (2017) Poly(N,N-dimethylacrylamide-co-acrylamide) grafted hydroxyethyl cellulose hydrogel: a useful Congo red dye remover. J Polym Environ 2017:1–18
Jeon YS, Lei J, Kim J-H (2008) Dye adsorption characteristics of alginate/polyaspartate hydrogels. J Ind Eng Chem 14:726–731
Jiang L, Liu P (2014) Design of magnetic attapulgite/fly ash/poly(acrylic acid) ternary nanocomposite hydrogels and performance evaluation as selective adsorbent for Pb2+ ion. ACS Sustain Chem Eng 2:1785–1794
Jin L, Bai R (2002) Mechanisms of lead adsorption on chitosan/pva hydrogel beads. Langmuir 18:9765–9770
Jing G, Wang L, Yu H, Amer WA, Zhang L (2013) Recent progress on study of hybrid hydrogels for water treatment. Colloids Surf A Physicochem Eng Asp 416:86–94
Joussein E, Petit S, Churchman J, Theng B, Righi D, Delvaux B (2005) Halloysite clay minerals a review. Clay Miner 40:383–426
Kabiri K, Omidian H, Zohuriaan-Mehr MJ, Doroudiani S (2011) Superabsorbent hydrogel composites and nanocomposites: a review. Polym Compos 2011:277–289
Kadirvelu K, Thamaraiselvi K, Namasivayam C (2001) Adsorption of nickel(II) from aqueous solution onto activated carbon prepared from coir pith. Sep Purif Technol 24:497–505
Kadirvelua K, Kavipriya M, Karthika C, Radhika M, Vennilamani N, Pattabhi S (2003) Utilization of various agricultural wastes for activated carbon preparation and application for the removal of dyes and metal ions from aqueous solutions. Bioresour Technol 87:129–132
Kamata H, Akagi Y, Kayasuga-Kariya Y, Chung U-i, Sakai T (2014) Nonswellable hydrogel without mechanical hysteresis. Science 343:873–875
Kasgoz H, Ozgumus S, Orbay M (2003) Modified polyacrylamide hydrogels and their application in removal of heavy metal ions. Polymer 44:1785–1793
Kasgoz H, Durmus A (2008) Dye removal by a novel hydrogel-clay nanocomposite with enhanced swelling properties. Polym Adv Technol 19:838–845
Khalil MI, Mostafa KM, Hebeish A (1993) Graft polymerization of acrylamide onto maize starch using potassium persulfate as initiator. Angew Makromol Chem 213:43–54
Kono H (2015) Preparation and characterization of amphoteric cellulose hydrogels as adsorbents for the anionic dyes in aqueous solutions. Gels 1:94–116
Kunz A, Mansilla H, Durán N (2002) A degradation and toxicity study of three textile reactive dyes by ozone. Environ Technol 23:911–918
Lagergren S (1898) Zur theorie der sogenannten adsorption gelöster stoffe, Kungliga Svenska Vetenskapsademiens. Handlingar 24:1–39
Langmuir I (1916) The constitution and fundamental properties of solids and liquids. J Am Chem Soc 38:2221–2295
Levis SR, Deasy PB (2002) Characterisation of halloysite for use as a microtubular drug delivery system. Int J Pharm 243:125–134
Li N, Bai R (2006) Highly enhanced adsorption of lead ions on chitosan granules functionalized with poly(acrylic acid). Ind Eng Chem Res 45:7897–7904
Li S (2010) Removal of crystal violet from aqueous solution by sorption into semi-interpenetrated networks hydrogels constituted of poly(acrylic acid-acrylamide-methacrylate) and amylose. Bioresour Technol 101:2197–2202
Li Z, Wang Y, Wu N, Chen Q, Wu K (2013) Removal of heavy metal ions from wastewater by a novel HEA/AMPS copolymer hydrogel: preparation, characterization, and mechanism. Environ Sci Pollut Res 20:1511–1525
Liu J, Su D, Yao J, Huang Y, Shao Z, Chen X (2017) Soy protein-based polyethylenimine hydrogel and its high selectivity for copper ion removal in wastewater treatment. J Mater Chem A 5:4163–4171
Lu Q, Yu J, Gao J, Yang W, Li Y (2011) Glow-discharge electrolysis plasma induced synthesis of polyvinylpyrrolidone/acrylic acid hydrogel and its adsorption properties for heavy-metal ions. Plasma Process Polym 8:803–814
Lvov YM, Shchukin DG, Mohwald H, Price RR (2008) Halloysite clay nanotubes for controlled release of protective agents. ACS Nano 2:814–820
Ma J, Zhou G, Chu L, Liu Y, Liu C, Luo S, Wei Y (2017) Efficient removal of heavy metal ions with an EDTA functionalized chitosan/polyacrylamide double network hydrogel. ACS Sustain Chem Eng 5:843–851
Magalhães ASG, Neto MPA, Bezerrac MN, Feitosa JPA (2013) Superabsorbent hydrogel composite with minerals aimed at water sustainability. J Braz Chem Soc 24:304–313
Mahmoud GA, Mohamed SF (2012) Removal of lead ions from aqueous solution using (sodium alginate/itaconic acid) hydrogel prepared by gamma radiation. Aust. J. Basic Appl Sci 6:262–273
Mahmoud GA, Mohamed SF, Hassan HM (2014) Removal of methylene blue dye using biodegradable hydrogel and reusing in a secondary adsorption process. J Desalin Water Treat 54:1–12
Mai NXD, Bae J, Kim T, Park SH, Lee G-W, Kim JH, Lee D, Son HB, Lee Y-C, Hur J (2017) A recyclable, recoverable, and reformable hydrogel-based smart photocatalyst. Environ Sci: Nano 4:955–966
Mirabedini M, Kassaee MZ, Poorsadeghi S (2017) Novel magnetic chitosan hydrogel film, cross-linked with glyoxal as an efficient adsorbent for removal of toxic Cr(VI) from water. Arab J Sci Eng 42:115–124
Mittal H, Maity A, Ray SS (2015) The adsorption of Pb2+ and Cu2+ onto gum ghatti-grafted poly(acrylamide-co-acrylonitrile) biodegradable hydrogel: isotherms and kinetic models. J Phys Chem B 119:2026–2039
Mohammadi Z, Shangbin S, Berkland C, J-t L (2017) Chelator-mimetic multi-functionalized hydrogel: highly efficient and reusable sorbent for Cd, Pb, and As removal from waste water. Chem Eng J 307:496–502
Mohammed N, Grishkewich N, Berry RM, Tam KC (2015) Cellulose nanocrystal–alginate hydrogel beads as novel adsorbents for organic dyes in aqueous solutions. Cellulose 22:3725–3738
Mohan SV, Sailaja P, Srimurali M, Karthikeyan J (1998) Color removal of monoazo acid dye from aqueous solution by adsorption and chemical coagulation. Environ Eng Policy 1:149–154
Moradi M, Kim JC, Qi J, Xu K, Li X, Ceder G, Belcher AM (2016) Bio-facilitated synthetic route for nano-structured complex electrode materials. Green Chem 18:2619–2624
Mostafa KM, El-Sanabary AA (2003) Graft polymerization of different monomers onto carbamated starches derived from native and hydrolyzed starches. J Appl Polym Sci 88:959–965
Nakata K, Fujishima A (2012) TiO2 photocatalysis. Design and applications. J Photochem Photobiol C 13:169–189
Ngah WSW, Endud CS, Mayanar R (2002) Removal of copper(II) ions from aqueous solution onto chitosan and cross-linked chitosan beads. React Funct Polym 50:181–190
Nguyen N-T, Liu J-H (2014) A green method for in situ synthesis of poly(vinyl alcohol)/chitosan hydrogel thin films with entrapped silver nanoparticles. J Taiwan Inst Chem Eng 45:2827–2833
Oladipo AA, Gazi M (2015) Microwaves initiated synthesis of activated carbon-based composite hydrogel for simultaneous removal of copper(II) ions and direct red 80 dye: a multi-component adsorption system. J Taiwan Inst Chem Eng 47:125–136
Pal P, Banat F (2015) Removal of contaminants from industrial lean amine solvent using polyacrylamide hydrogels optimized by response surface methodology. Adsorpt Sci Technol 33:9–24
Patel AM, Patel RG, Patel MP (2010) Nickel and copper removal study from aqueous solution using new cationic poly[acrylamide/N,N-DAMB/N,N-DAPB] super absorbent hydrogel. J Appl Polym Sci 119:2485–2493
Pathak SK, Dwivedi C, Banerjee C, Singh KK, Tripathi SC, Kumar M, Gandhi PM (2016) Potassium zinc hexacyanoferrate encapsulated hydro-gel beads for removal of radio-cesium. Asian J Mater Chem 1:14–20
Paulino AT, Belfiore LA, Kubota LT, Muniz EC, Almeida VC, Tambourgi EB (2011) Effect of magnetite on the adsorption behavior of Pb(II), Cd(II), and Cu(II) in chitosan-based hydrogels. Desalination 275:187–196
Peng N, Hu D, Zeng J, Li Y, Liang L, Chang C (2016) Superabsorbent cellulose–clay nanocomposite hydrogels for highly efficient removal of dye in water. ACS Sustain Chem Eng 4:7217–7224
Peng Q, Liu M, Zheng J, Zhou C (2015) Adsorption of dyes in aqueous solutions by chitosan–halloysite nanotubes composite hydrogel beads. Microporous Mesoporous Mater 201:190–201
Pérez S, Mazeau K, CHd P (2000) The three-dimensional structures of the pectic polysaccharides. Plant Physiol Biochem 38:37–55
Petzold G, Schwarz S (2014) Polyelectrolyte complexes in flocculation applications. Adv Polym Sci 256:25–66
Philipp B, Dautzenberg H, K-j L, Kotz J, Dawydoff W (1989) Polyelectrolyte complexes—recent developments and open problems. Prog Polym Sci 14:91–172
Pour ZS, Ghaemy M (2015) Removal of dyes and heavy metal ions from water by magnetic hydrogel beads based on poly(vinyl alcohol)/carboxymethyl starch-g-poly(vinyl imidazole). RSC Adv 5:64106–64118
Pourjavadi A, Salimi H, Amini-Fazl MS, Kurdtabar M, Amini-Fazl AR (2006) Optimization of synthetic conditions of a novel collagen-based superabsorbent hydrogel by taguchi method and investigation of its metal ions adsorption. J Appl Polym Sci 102:4878–4885
Pourjavadi A, Tehrani ZM, Salimi H, Banazadeh A, Abedini N (2015) Hydrogel nanocomposite based on chitosan-g-acrylic acid and modified nanosilica with high adsorption capacity for heavy metal ion removal. Iran Polym J 24:725–734
Purkait MK, Maiti A, DasGupta S, De S (2007) Removal of Congo red using activated carbon and its regeneration. J Hazard Mater 145:287–295
Qada ENE, Allen SJ, Walker GM (2006) Adsorption of methylene blue onto activated carbon produced from steam activated bituminous coal: a study of equilibrium adsorption isotherm. Chem Eng J 124:103–110
Ralph J, Lundquist K, Brunow G (2004) Lignins: natural polymers from oxidative coupling of 4-hydroxyphenyl-propanoids. Phytochem Rev 3:29–60
Redlich O, Peterson DL (1959) A useful adsorption isotherm. J Phys Chem 63:1024
Reis AV, Guilherme MR, Paulino AT, Muniz EC, Mattoso LHC, Tambourgi EB (2009) Synthesis of hollow-structured nano- and microspheres from pectin in a nanodroplet emulsion. Langmuir 25:2473–2478
Reis LA, Chiu LLY, Liang Y, Hyunh K, Momen A, Radisic M (2012) A peptide-modified chitosan–collagen hydrogel for cardiac cell culture and delivery. Acta Biomater 8:1022–1036
Rocher V, Siaugue J-M, Cabuil V, Bee A (2008) Removal of organic dyes by magnetic alginate beads. Water Res 42:1290–1298
Rossi F, Motta O, Matrella S, Proto A, Vigliotta G (2015) Nitrate removal from wastewater through biological denitrification with OGA 24 in a batch reactor. Water 7:51–62
Schneider J, Matsuoka M, Takeuchi M, Zhang J, Horiuchi Y, Anpo M, Bahnemann DW (2014) Understanding TiO2 photocatalysis. Mechanisms and materials. Chem Rev 114:9919–9986
Sebesta F, John J, Motl A, Stamberg K (1995) Evaluation of polyacrylonitrile (PAN) as a binding polymer for absorbers used to treat liquid radioactive wastes. Technical Report in Sandia National Labs. SciTech Connect, Albuquerque, pp 1–46
Senkal BF, Erkal D, Yavuz E (2006) Removal of dyes from water by poly(vinyl pyrrolidone) hydrogel. Polym Adv Technol 17:924–927
Sethuraman VV, Raymahashay BC (1975) Color removal by clays. Kinetic study of adsorption of cationic and anionic dyes. Environ. Sci. Technol. 9:1139–1140
Singh V, Joung D, Zhai L, Das S, Khondaker SI, Seal S (2011) Graphene based materials: past, present and future. Prog Mater Sci 56:1178–1271
Sips R (1948) Combined form of Langmuir and Freundlich equations. J Chem Phys 16:490–495
Souda P, Sreejith L (2014) Environmental sensitive hydrogel for purification of waste water: part 1: synthesis and characterization. Polym Bull 71:839–854
Srivastav RK, Gupta SK, Nigam KDP, Vasudevan P (1994) Treatment of chromium and nickel in wastewater by using aquatic plants. Water Res 28:1631–1638
Suna S, Wanga L, Wang A (2006) Adsorption properties of crosslinked carboxymethyl-chitosan resin with Pb(II) as template ions. J Hazard Mater 136:930–937
Tang SCN, Wang P, Yin K, Lo IMC (2010) Synthesis and application of magnetic hydrogel for Cr(VI) removal from contaminated water. Environ Eng Sci 27:947–954
Tang SCN, Yan DYS, Lo IMC (2014) Sustainable wastewater treatment using microsized magnetic hydrogel with magnetic separation technology. Ind Eng Chem Res 53:15718–15724
Thakura VK, Thakurb MK (2015) Recent advances in green hydrogels from lignin: a review. Int J Biol Macromol 72:834–847
Thünemann AF, Müller M, Dautzenberg H, Joanny J, Löwen H (2004) Polyelectrolyte complexes. Adv Polym Sci 166:113–171
Trakulsujaritchok T, Noiphom N, Tangtreamjitmun N, Saeeng R (2011) Adsorptive features of poly(glycidyl methacrylateco-hydroxyethyl methacrylate): effect of porogen formulation on heavy metal ion adsorption. J Mater Sci 46:5350–5362
Tu H, Yu Y, Chen J, Shi X, Zhou J, Deng H, Du Y (2017) Highly cost-effective and high-strength hydrogels as dye adsorbents from natural polymers: chitosan and cellulose. Polym Chem 8:2913–2921
Vieira RS, Beppu MM (2006) Dynamic and static adsorption and desorption of Hg(II) ions on chitosan membranes and spheres. Water Res 40:1726–1734
Vimonses V, Lei S, Jin B, Chow CWK, Saint C (2009) Kinetic study and equilibrium isotherm analysis of Congo red adsorption by clay materials. Chem Eng J 148:354–364
Vivek B, Prasad E (2015) Reusable self-healing hydrogels realized via in situ polymerization. J Phys Chem B 119:4881–4887
Vrtoch L, Pipıska M, Hornık M, Augustın J, Lesny J (2011) Sorption of cesium from water solutions on potassium nickel hexacyanoferrate-modified Agaricus bisporus mushroom biomass. J Radioanal Nucl Chem 287:853–862
Wang H, Li X, Chai L, Zhang L (2015) Nano-functionalized filamentous fungus hyphae with fast reversible macroscopic assembly & disassembly features. Chem. Comm. 51:8524–8527
Wang L, Wang A (2008) Adsorption properties of Congo red from aqueous solution onto N,O-carboxymethyl-chitosan. Bioresour Technol 99:1403–1408
Wang N, Han Y, Liu Y, Bai T, Gao H, Zhang P, Wang W, Liu W (2012) High-strength hydrogel as a reusable adsorbent of copper ions. J Hazard Mater 213-214:258–264
Wang S, Wu H (2006) Environmental-benign utilisation of fly ash as low-cost adsorbents. J Hazard Mater 136:482–501
Wang W, Wang A (2010) Synthesis and swelling properties of pH-sensitive semi-IPN superabsorbent hydrogels based on sodium alginate-g-poly(sodium acrylate) and polyvinylpyrrolidone. Carbohydr Polym 80:1028–1036
Wang X, Hou H, Li Y, Wang Y, Hao C, Ge C (2016) A novel semi-IPN hydrogel: preparation, swelling properties and adsorption studies of Co (II). J Ind Eng Chem 41:82–90
Wang Y, Wang W, Shi X, Wang A (2013) A superabsorbent nanocomposite based on sodium alginate and illite/smectite mixed-layer clay. J Appl Polym Sci 130:161–167
Weber W, Morris J (1963) Kinetics of adsorption on carbon from solution. J Sanit Eng Div 89:31–60
Weng C-H, Pan Y-F (2006) Adsorption characteristics of methylene blue from aqueous solution by sludge ash. Colloids Surf A Physicochem Eng Asp 274:154–162
Wibulswas R (2004) Batch and fixed bed sorption of methylene blue on precursor and QACs modified montmorillonite. Sep Purif Technol 39:3–12
Willats WGT, Knox JP, Mikkelsen JD (2006) Pectin: new insights into an old polymer are starting to gel. Trends Food Sci Technol 17:97–104
Wu N, Li Z (2013) Synthesis and characterization of poly(HEA/MALA) hydrogel and its application in removal of heavy metal ions from water. Chem Eng J 215-216:894–902
Yagub MT, Sen TK, Afroze S, Ang HM (2014) Dye and its removal from aqueous solution by adsorption: a review. Adv Colloid Interf Sci 209:172–184
Yan H, Yang L, Yang Z, Yang H, Li A, Cheng R (2012) Preparation of chitosan/poly(acrylic acid) magnetic composite microspheres and applications in the removal of copper(II) ions from aqueous solutions. J Hazard Mater 229-230:371–380
Yang S, Fu S, Liu H, Zhou Y, Li X (2010) Hydrogel beads based on carboxymethyl cellulose for removal heavy metal ions. J Appl Polym Sci 119:1204–1210
Yavuz CT, Prakash A, Mayoa JT, Colvin VL (2009) Magnetic separations: from steel plants to biotechnology. Chem Eng Sci 64:2510–2521
Yoshida H, Takemori T (1997) Adsorption of direct dye on cross-linked chitosan fiber: breakthrough curve. Water Sci Technol 35:29–37
Yu C, Wang F, Zhang C, Fu S, Lucia LA (2016) The synthesis and absorption dynamics of a lignin-based hydrogel for remediation of cationic dye-contaminated effluent. React Funct Polym 106:137–142
Yu L, Liu X, Yuan W, Brown LJ, Wang D (2015a) Confined flocculation of ionic pollutants by poly(l-dopa)-based polyelectrolyte complexes in hydrogel beads for three-dimensional, quantitative, efficient water decontamination. Langmuir 31:6351–6366
Yu Y, Andrade LCXD, Fang L, Ma J, Zhang W, Tang Y (2015b) Graphene oxide and hyperbranched polymer-toughened hydrogels with improved absorption properties and durability. J Mater Sci 50:3457–3466
Zenk MH (1996) Heavy metal detoxification in higher plants—a review. Gene 179:21–30
Zhang B, Cui Y, Yin G, Li X (2012a) Adsorption of copper (II) and lead (II) ions onto cottonseed protein-paa hydrogel composite. Polym Plast Technol Eng 51:612–619
Zhang L, Chai L, Liu J, Wang H, Yu W, Sang P (2011) pH manipulation: a facile method for lowering oxidation state and keeping good yield of poly(m-phenylenediamine) and its powerful Ag+ adsorption ability. Langmuir 27:13729–13738
Zhang L, Wang H, Yu W, Su Z, Chai L, Lia J, Shia Y (2012b) Facile and large-scale synthesis of functional poly(m-phenylenediamine) nanoparticles by Cu2+-assisted method with superior ability for dye adsorption. J Mater Chem 22:18244–18251
Zhang L, Li X, Wang M, He Y, Chai L, Huang J, Wang H, Wu X, Lai Y (2016) Highly flexible and porous nanoparticle-loaded films for dye removal by graphene oxide-fungus interaction. ACS Appl Mater Interfaces 8:34638–34647
Zhang L, Sun J (2009) Layer-by-layer deposition of polyelectrolyte complexes for the fabrication of foam coatings with high loading capacity. Chem Comm 3091–390. https://doi.org/10.1039/b907691c
Zhao L, Mitomo H (2008) Adsorption of heavy metal ions from aqueous solution onto chitosan entrapped CM-cellulosehydrogels synthesized by irradiation. J Appl Polym Sci 110:1388–1395
Zhao LZ, Zhou CH, Wang J, Tong DS, Yua WH, Wang H (2015) Recent advances in clay mineral-containing nanocomposite hydrogels. Soft Matter 11:9229–9246
Zheng Y, Hua S, Wang A (2010) Adsorption behavior of Cu2+ from aqueous solutions onto starch-g-pol (acrylic acid)/sodium humate hydrogels. Desalination 263:170–175
Zheng Y, Huanga D, Wang A (2011) Chitosan-g-poly(acrylic acid) hydrogel with crosslinked polymeric networks for Ni2+ recovery. Anal Chim Acta 687:193–200
Zhixin J, Baochun G, Demin J (2014) Advances in rubber/halloysite nanotubes nanocomposites. J Nanosci Nanotechnol 14:1758–1771
Zhou J, Zhang L, Deng Q, Wu X (2004) Synthesis and characterization of cellulose derivatives prepared in NaOH/urea aqueous solutions. J Polym Sci A Polym Chem 42:5911–5920
Zhou W, Xiong T, Shi C, Zhou J, Zhou K, Zhu N, Li L, Tang Z, Chen S (2016) Bioreduction of precious metals by microorganism: efficient gold@n-doped carbon electrocatalysts for the hydrogen evolution reaction. Angew Chem Int Ed 55:8416–8420
Zhou Y, Fu S, Liu H, Yang S, Zhan H (2011) Removal of methylene blue dyes from wastewater using cellulose-based superadsorbent hydrogels. Polym Eng Sci 51:2417–2424
Zhou Y, Zhang L, Fu S, Zheng L, Zhan H (2012) Adsorption behavior of Cd2+, Pb2+, and Ni2+ from queous solutions on cellulose-based hydrogels. Bioresources 7:2752–2765
Zhu S, Wu Y, Chen Q, Yu Z, Wang C, Jin S, Ding Y, Wuc G (2006) Dissolution of cellulose with ionic liquids and its application: a mini-review. Green Chem 8:325–327
Zhu W-K, Cong H-P, Guan Q-F, Yao W-T, Liang H-W, Wang W, Yu S-H (2016) Coupling microbial growth with nanoparticles: a universal strategy to produce functional fungal hyphae macrospheres. ACS Appl Mater Interfaces 8:12693–12701
Zhuang Y, Yu F, Chen H, Zheng J, Ma J, Chen J (2016) Alginate/graphene double-network nanocomposite hydrogel beads with low-swelling, enhanced mechanical properties, and enhanced adsorption capacity. J Mater Chem A 4:10885–10892
Zollinger H (2003). Color chemistry: synthesis, properties, and applications of organic dyes and pigments, 3rd revised edition. VHCA-Verlag Helvetica Chimica Acta and Wiley-VCH, 637 pp
Funding
This research was supported by a grant from R&D Program of the Korea Railroad Research Institute (KRRI) and by the Basic Science Research Program through the National Research Foundation of Korea funded by the Ministry of Education (NRF-2017R1D1A1A09000642) and by the Korean Ministry of Environment’s GAIA project (2015000550006).
Author information
Authors and Affiliations
Corresponding author
Additional information
Responsible editor: Angeles Blanco
Highlights
• Unique particles entrapped in synthesis process of three types of hydrogels
• Adsorption kinetic models, adsorption isotherm models, and the mechanism of contaminants on hydrogels
• Impact factors on the adsorption capacity of hydrogels: pH, adsorbate concentration, swelling degree, and other ions
• Recycle and recovery methods of used hydrogels and effect of mechanical properties on the reusability of hydrogels
• Applications of hydrogels on removal of metal ion, dye, and radionuclides from water/wastewater
Rights and permissions
About this article
Cite this article
Van Tran, V., Park, D. & Lee, YC. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ Sci Pollut Res 25, 24569–24599 (2018). https://doi.org/10.1007/s11356-018-2605-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11356-018-2605-y